Abstract

Background: Scoring systems at CML diagnosis, such as Sokal risk, provide important response prediction for imatinib (IM) treated patients (pts). Specific treatment policies have been suggested for high risk pts to optimize otherwise inferior outcomes. However, responses among pts with high risk are heterogeneous and new biomarkers are required to facilitate rational selection of optimal therapy. Biological factors, such as germline genetic variation, may play a role in therapy response dynamics. We aimed to identify predictive biomarkers of response to IM at CML diagnosis to aid selection of front line therapy for optimal treatment outcomes.
Methods: Targeted amplicon sequencing using a custom Ion AmpliSeq panel and the Ion Proton was performed for 35 genes: 10 BCL2 family genes involved in TKI initiated apoptosis (including BIM, BAD and BCL2); 5 drug metabolism genes; and 20 genes implicated in hematologic malignancies (including ASXL1 and TET2). Genotypes were determined for 200 candidate single nucleotide variants (SNPs) for 528 front line IM and 83 front line NIL treated pts. For the IM pts, baseline variables were assessed for association with outcome: Sokal risk, age, gender, assigned IM dose (400, 600 or 800 mg); and genotype.
Results: SNPs significantly associated with outcome in univariate analyses were assessed in multivariate models with the other baseline variables. The Sokal risk, ASXL1 rs4911231 and BIM rs686952 SNPs were independent predictors of 12 mo MMR, 48 mo MR4, MR4.5 and failure free survival (FFS, loss of any response, death, progression to AP/BC). For the ASXL1 SNP, the homozygous T genotype (155/508 evaluable pts, 30%), and for the BIM SNP, the A allele (249/507 evaluable pts, 49%) were associated with superior outcomes.
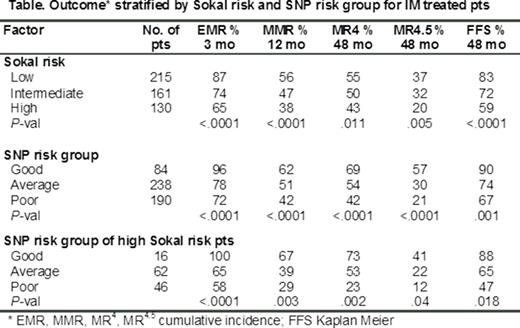
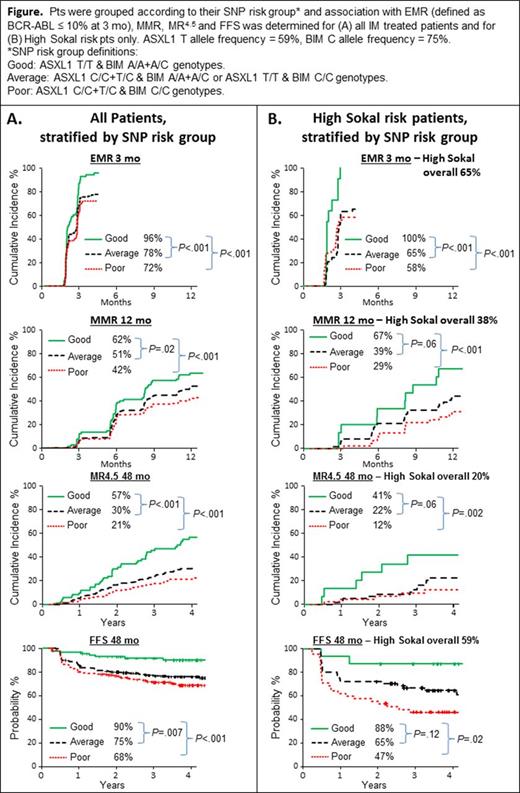
We explored the additive effect of combining the genotypes of the ASXL1 and BIM SNPs on outcome. Three risk groups were readily identified (defined in Fig): Good (16% of evaluable pts), Average (46%) and Poor (37%). There were significant differences in the cumulative incidence of 3 mo EMR, 12 mo MMR, and 48 mo MR4, MR4.5 and FFS, as stratified by these SNP risk groups in IM treated pts (Table and Fig A). No significant association was found for progression to AP/BC or survival for any baseline variable.
To examine the predictive power of SNP genotype within the high Sokal risk group, high risk pts were stratified by SNP genotype group. Significant differences were observed for EMR, MMR, MR4, MR4.5 and FFS (Table and Figure B), demonstrating the ability of the SNP genotype within high Sokal risk pts to predict response. Moreover, high Sokal risk pts harboring a poor risk SNP genotype had a significantly higher risk of progression to AP/BC vs high Sokal risk pts with an average/good risk genotype, 12% vs 2% (P =.03).
The impact of SNP genotype risk on achieving 12 mo MMR was examined in the 83 pts treated with frontline NIL (median 24 mo follow up). In contrast to the significant difference observed for IM pts, there was no significant difference for NIL pts: 75% vs 73% vs 64% for good, average and poor risk, respectively, P =.34, suggesting the poor risk conferred by genotype may be abrogated by more potent TKI.
Conclusion: Our data suggest inherent genetic variation contributes to the heterogeneity of response to IM. An intronic SNP in BIM, a key initiator of TKI induced apoptosis, and a synonymous SNP in ASXL1 exon 12, a region commonly mutated in hematologic cancers, were strong biomarkers of IM response. The mechanism by which these SNPs affect response awaits further clinical and experimental evaluation. Among pts with high Sokal risk, the genotype of these 2 SNPs delineated response and identified a good risk subgroup where more potent TKI may not be required for optimal outcomes. Assessment of genetic variation at diagnosis may contribute to a prognostic score that will allow for optimization of therapy.
Yeung:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grant international meeting, Research Funding. Hughes:Bristol-Myers Squibb: Honoraria, Research Funding; ARIAD: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Branford:BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Qiagen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal