Abstract
Introduction: The receptor tyrosine kinase (RTK) insulin-like growth factor-1 receptor (IGF1R) is dysregulated in various tumor entities and hematological malignancies including chronic lymphocytic leukemia and mantle cell lymphoma. The implication of IGF1R in the development and progression of cancer has led to its current evaluation in clinical trials as a potential therapeutic target for solid tumors. However, its functional significance in diffuse large B-cell lymphoma (DLBCL) remains poorly characterized. We hypothesized that IGF1R plays a key role in the pathogenesis and progression of DLBCL. In this present study, we evaluated the expression and function of IGF1R in both B cell lines and DLBCL tissues, as well as assessed the proliferation and apoptosis of DLBCL cells when treated with IGF-1R inhibitor, AG1024.
Methods: Expression of IGF1R in B-cell lymphoma cell lines (LY1, LY8, Mino, Jeko-1, and SP53) was evaluated by Western blotting. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers with informed consents. Blood samples and araffin-embedded tissues from 30 initial-diagnosed DLBCL patients prior to therapeutic interventions as a study group, and from 15 patients with reactive hyperplasia lymphnode as a control group were collected with informed consents. Immunohistochemisty (IHC) was conducted to assess the expression of IGF-1R in lymphoma tissues. Correlations between IGF1R expression and the clinical characteristics of DLBCL patients were further analyzed. DLBCL cell lines (LY1 and LY8) were treated with an IGF1R specific small molecular inhibitor, AG1024, cell proliferation was analyzed by cell counting kit (CCK-8). Effects of inhibitor or stimulator on the apoptosis of LY1 and LY8 cells were assessed by Annexin-V/PI and Annexin-V/7AAD, respectively. Expression of apoptosis-related protein, including Caspase-3 and Mcl-1, was evaluated by western blotting. Protein levels of downstream targets of IGF-1 signaling were also detected.
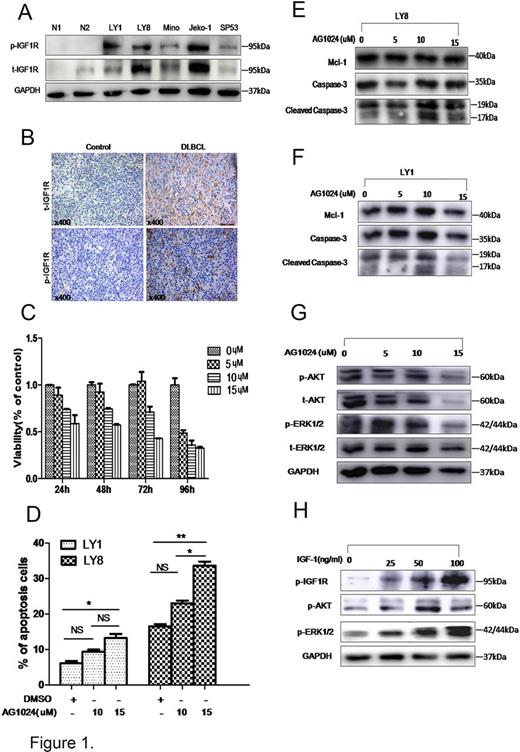
Results: Significantly upregulation of both phoaphprylated and total IGF1R protein levels were found in B-cell lymphoma cells (LY1, LY8, Mino, Jeko-1 and SP53) (Fig 1.A). IHC was conducted and revealed significantly enhancement of IGF1R expression in DLBCL patients (Fig 1.B). Among the included DLBCL patients and control group with inreactive hyperplastic lymphadenitis, the positive rate of IGF1R was 90% and 20%, respectively. We then investigated the function of IGF1R inhibitors on the proliferation and apoptosis of DLBCL cells. LY8 cells were treated with different doses of AG1024 at 24-96 hours. Cell proliferation was inhibited by 60% when treated with AG1024 at the concentration of 15µM for 72-hours (Fig 1.C). Culture of LY1 and LY8 cells in the presence of 10µM and 15µM AG1024 concentration for 24-hours resulted in 13% (p<0.05) and 33% (p<0.001) cell apoptosis, respectively (Fig 1.D). Inhibition of IGF1R by AG1024 also resulted in induction of cleaved-Caspase-3, as well as reduction of Mcl-1(Fig 1.E-F). In order to investigate the mechanisms involved in the dysregultaion of IGF1R in DLBCL, LY8 cells were treated with 5 to 15 µM AG1024, the results revealed that AG1024 caused a dose-dependent decrease in the levels of phosphorylated IGF1R, AKT and ERK (Fig 1.G). Treatment of LY8 cells with recombinant human IGF-1 led to enhanced phosporylation levels of IGF1R, AKT and ERK (Fig 1.H).
Conclusions: Our investigation observed that expression levels of IGF-1R were up-regulated in both B-cell lymphoma cells and DLBCL tissues. DLBCL cells treated with IGF-1R inhibitor, AG1024, revealed reduced proliferation and increased apoptosis rate. In addition, induction of cleaved-Caspase-3 was also found in LY1 treated with AG1024. AG1024 caused a dose-dependent decrease in the phosphorylation levels of IGF1R, AKT and ERK. This study suggests that IGF1R could be a potential molecular target for the treatment of DLBCL. The IGF-1R inhibitor is a promising therapeutic approach for DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal