Abstract
Background: We have overcome the limitations of 40 years of ex vivo testing. The aim of this study is to determine the ability of Vivia's novel test to predict the complete remission (CR) rates after induction chemotherapy with cytarabine (Ara-C) and idarubicin (Ida) in 1st line AML.
Material and Methods: Bone marrow samples from adult patients diagnosed with de novo AML in Spanish centers from the PETHEMA group were included. Whole marrow samples maintaining their Native Environment were incubated for 48h in well plates containing Ara-C, Ida, or their combination. Pharmacological responses are calculated using population models. Induction response was assessed according to the Cheson criteria (2003). Patients attaining a CR/CRi were classified as responders and the remaining as resistant.
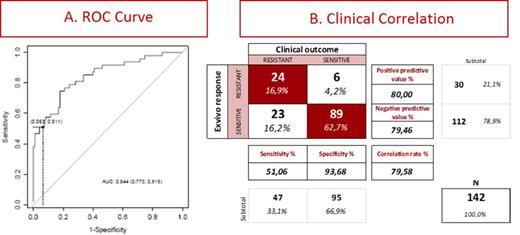
Results: 390 patient samples were used to calculate the dose response (DR) curves for Ara-C alone, Ida alone, and their synergism. For clinical correlation we used 142 patients with median 56 years. The strongest clinical predictor was the Area Under the Curve (AUC) of the DR of Ara-C, and the AUC of IDA. The GAM models revealed a significant relationship between the AUC of the concentration-effect curves of both, idarubicin and, particularly, Ara-C, with greater values associated to higher probabilities of post-induction resistance. The fitted Generalized Additive Method predictions of expected values for each patient were in turn related to overall survival when a discrimination value to define positive and negative test results that prioritized specificity over sensitivity was chosen based on equaling positive and negative predictive values (Fig 1A). Prioritizing specificity over sensitivity reflects the higher cost of false positive over false negative decisions: only in very rare instances, an effective treatment would be erroneously negated to a sensitive patient at the expense of overlooking a number of resistant patients. However, the later patients could take their chances on re-induction therapy. While for diagnostics sensitivity and specificity should both be optimized, for Personalized Medicine the positive and negative predictive values should be optimized preferentially because they define the patient response correlation.
Fig 1B shows a table illustrating the correlation between clinical outcome (columns) and the test predictions (lines). From a diagnostic criteria (columns), clinically resistant patients (1st column) are not well predicted with a Sensitivity of 51%, while clinically sensitive patients (2nd column) are very well predicted with a Specificity of 94%. From a Precision Medicine criteria (Lines), patients predicted resistant (1st line) and well predicted with 80% positive predictive value, similar to patients predicted sensitive (2nd line) well predicted with 79% Negative Predictive Value.
The test does not properly identify 23/142 that are clinically resistant and the test predicts as sensitive (bottom left quadrant right panel). This mismatched subgroup mimics the problems from molecular markers where a resistant clone present in a minority of leukemic cells cannot be detected yet drives the patient response. However, this group mismatch does not prevent a good correlation with the test predicted outcomes. Flow cytometry identified 2 clones in 75% of these 23 samples, and we revised all samples analyzing each of 2 clones separately whenever they were present. Results did not change by this clonal analysis, suggesting flow cytometry may not identify resistant clones. Future improvements of the test adding 16 concentrations to the dose response curves may be able to detect the presence and parameters of these resistant clones driving patient response.
Conclusions: This novel test is able to predict the clinical response to Ida + Ara-C induction with overall correlation and predictive values of 80%, higher than ever achieved. It is significantly higher than the current clinical response rate of 66.7%. Thus this novel test may be valuable information to guide 1st line patient therapy.
Ballesteros:Vivia Biotech: Employment. Cordoba:Celgene: Research Funding. Ramos:GlaxoSmithKline: Honoraria; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria; Celgene Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria. Gaspar:Vivia Biotech: Employment. Gorrochategui:Vivia Biotech: Employment. Rojas:Vivia Biotech: Employment. Gomez:Vivia Biotech: Employment. Hernández:Vivia Biotech: Employment. Robles:Vivia Biotech: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal