Abstract
Granulocyte colony-stimulating factor (G-CSF) has been known to reduce the duration of neutropenia and the incidence of infection-related death in patients with solid cancer who were treated by cytotoxic chemotherapies. A few randomized trials addressed these benefits in patients with non-M3 acute myelogenous leukemia (AML). However, long-term effects of G-CSF exposure in these patients were not reported. We analyzed the impact of G-CSF for outcomes of non-M3 AML patients who were treated by anthracycline-based induction and high-dose cytarabine consolidation chemotherapies.
The patients enrolled in the Korea University AML registry from September 2002 to December 2014 were analyzed. AML patients who were treated by anthracycline-based inducton (7+3 regimen) and high-dose cytarabine consolidation (cytarabine 3g/m2 q 12 hours on D1, 3, 5) chemotherapies were included for this analysis. Patients with acute promyelocytic leukemia were excluded. The enrolled patients were classified into 3 subgroups based on G-CSF administration strategies during induction chemotherapies; 1) patients without G-CSF exposure during induction (no G-CSF group), 2) patients with G-CSF administration which was initiated immediately after development of neutropenia (absolute neutrophil counts < 1000) but before development of febrile neutropenia (preemptive group), 3) patients with G-CSF administration which was initiated after development of febrile neutropenia (therapeutic group).
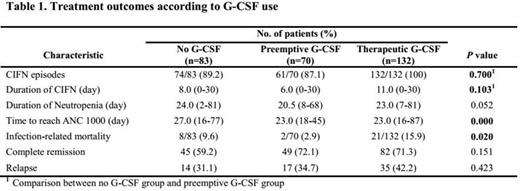
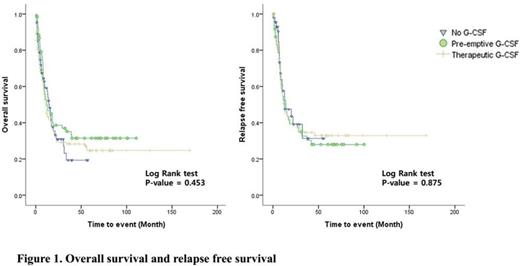
285 patients were included in this analysis. The median follow-up duration was 18 months. The number of no G-CSF, preemptive and therapeutic group were 83, 70, and 132, respectively. The impacts of G-CSF administration strategies for treatment outcomes were summarized in table 1. The incidence and duration of chemotherapy-induced febrile neutropenia (CIFN) was not statistically different in no G-CSF group and preemptive group (p=0.700 and p=0.103, respectively). According to the median duration of neutropenia, preemptive G-CSF group (20.5 days) showed marginal benefit compared to the other groups (24 days in no G-CSF group and 23 days in therapeutic group) (p=0.052). The median time to ANC recovery (>1000) was significantly shorter in preemptive group (23 days) and therapeutic group (23 days) compared to no G-CSF group (27 days) (p<0.001). Infection-related mortality was significantly lower in preemptive group (2.9%) compared to no G-CSF group (9.6%) and therapeutic group (15.9%) (p=0.020). Complete remission rate and cumulative incidence of relapse were not significantly different between groups (p=0.151 and p=0.423, respectively). In multivariate analysis for incidence of CIFN, quinolone prophylaxis was independently significant factor for reducing the incidence of CIFN (HR 0.222, 95% confidence interval 0.054-0.911, p=0.037). In multivariate analysis for infection-related death, young age (°Â60) and preemptive group were independently significant factors for reducing the development of infection-related death (HR 0.284, 95% confidence interval 0.120-0.676, p=0.004 and HR 0.108, confidence interval 0.012-0.934, p=0.043 respectively). According to the survival, there were no significant differences among groups in overall survival and relapse free survival (figure 1).
Preemptive G-CSF administration during induction treatments in non-M3 AML patients was considered to be effective in reducing infection-related deaths without affecting remission, relapse and overall survival. Because quinolone prophylaxis was showed to be effective in reducing the incidence of CIFN, combination of preemptive G-CSF administration and quinolone prophylaxis might be considered to provide synergistic benefit in non-M3 patients with intensive chemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal