Abstract
Introduction
In the treatment of acute myeloid leukemia, initial induction guidelines frequently include performing a day 14 bone marrow biopsy to assess response. A second induction is then recommended for patients with morphologic residual leukemia (≥5% blasts). Recent retrospective reviews have shown no difference in complete response (CR) rates amongst patients with residual leukemia based on day 14 marrow assessment who were and were not given a second induction. In our single center retrospective study, we compared patients with residual blast counts ≥5% to identify differences in leukemia free survival (LFS) and overall survival (OS) in those who did and did not receive a second induction. In addition we examined the cytogenetic profile of Day 14 and remission bone marrows.
Methods:
Consecutively treated patients with newly diagnosed primary and secondary AML over the age of 18 who received daunorubicin based induction with a curative intent from 2006 to 2014 were obtained via cancer center medical records. Outcome data was followed until May 1st 2015. We reviewed demographic (age, sex, ECOG PS, co-morbidities) as well as AML specific data (daunorubicin dose, use of etoposide, initial bone marrow blast count, initial white blood cell count, bone marrow blast count at day 14, cytogenetic stratification, CR rate, allogeneic stem cell transplant, LFS and OS). Patients included in survival analysis were required to have an initial and day 14 bone marrow biopsy with morphologic identification of residual leukemia on the day 14 marrow. Cytogenetic risk stratification was based on NCCN guidelines. Morphological CR was defined based on International Working Group criteria (Cheson, JCO, 2003). Positive (PPV) and negative predictive value (NPV) for CR were calculated by considering a BM of less than 5% at Day 14 a positive screening test for obtaining CR. Data was analyzed using Mann Whitney U test and chi square analysis as appropriate. LFS and OS were estimated using the Kaplan Meier method and compared using the log rank test. All calculations and graphs were obtained using STATA/SE 12.1.
Results:
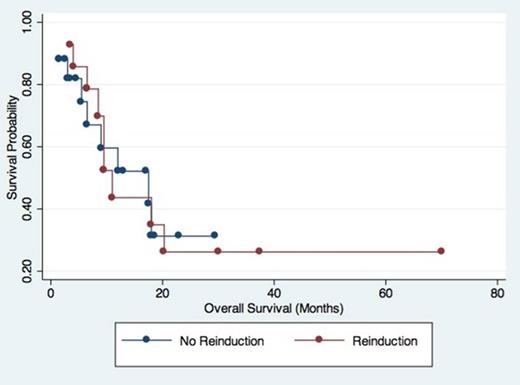
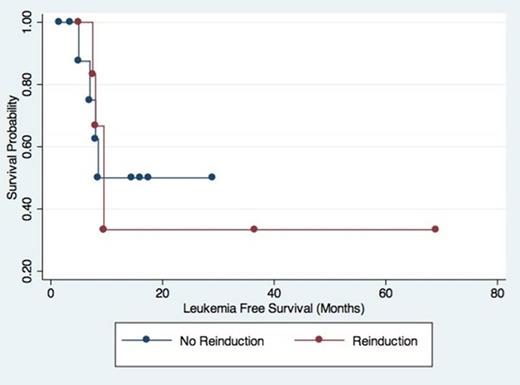
Of the 91 patients initially identified, 67 had Day 14 marrows performed and 31 had residual blast counts ≥5%. For our overall cohort, we found a PPV of 76% and NPV of 65% for Day 14 marrows in determination of CR, which is in line with previous studies. No statistical differences were seen between the groups (Table 1), although patients who were reinduced trended toward higher initial white blood cell counts while those who were not reinduced trended towards improved CR rates. Comparing patients with residual disease at Day 14 who were or were not reinduced, we found no statistical difference for LFS and OS between the two groups (p 0.87 and 0.96 respectively). Interestingly, in comparing cytogenetics, two patients with unfavorable cytogenetics on initial BM converted to normal/intermediate cytogenetics after induction despite a residual blast count greater than 5%. Both obtained CR, one with and one without reinduction. Another patient with unfavorable cytogenetics at day 14 obtained CR with normal cytogenetics without reinduction.
Conclusions:
Although of limited size, our study suggests that reinduction based on Day 14 marrow is of limited use in predicting LFS and OS in AML. Better methods to determine residual leukemia following initial induction therapy are required in order to help guide treatment decisions.
Demographics of patients with and without reinduction chemotherapy with residual disease on repeat bone marrow biopsy
| . | No Reinduction (n=17) . | Reinduction (n=14) . | p value . |
|---|---|---|---|
| Age (Median) | 63 | 57.5 | 0.46 |
| Male:Female | 8:9 | 8:6 | 0.58 |
| ECOG PS 0 1 | 11 6 | 10 4 | 0.69 |
| Initial WBC (Median) | 5.2 | 11.7 | 0.06 |
| Initial % Bone Marrow Blast (Median) | 0.53 | 0.48 | 0.56 |
| DNR Dose 60mg/m2 90mg/m2 | 11 6 | 8 6 | 0.67 |
| Etoposide Given | 5 | 4 | 0.96 |
| Cytogenetic Risk Intermediate Unfavorable | 9 8 | 7 7 | 0.87 |
| Complete Response | 10 | 4 | 0.09 |
| Allo-SCT | 7 | 8 | 0.38 |
| . | No Reinduction (n=17) . | Reinduction (n=14) . | p value . |
|---|---|---|---|
| Age (Median) | 63 | 57.5 | 0.46 |
| Male:Female | 8:9 | 8:6 | 0.58 |
| ECOG PS 0 1 | 11 6 | 10 4 | 0.69 |
| Initial WBC (Median) | 5.2 | 11.7 | 0.06 |
| Initial % Bone Marrow Blast (Median) | 0.53 | 0.48 | 0.56 |
| DNR Dose 60mg/m2 90mg/m2 | 11 6 | 8 6 | 0.67 |
| Etoposide Given | 5 | 4 | 0.96 |
| Cytogenetic Risk Intermediate Unfavorable | 9 8 | 7 7 | 0.87 |
| Complete Response | 10 | 4 | 0.09 |
| Allo-SCT | 7 | 8 | 0.38 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal