Abstract
Backround: The treatment of mycosis fungoides (MF) is determined by disease extent, prognostic factors and patient characteristics. Extracorporeal Photopheresis (ECP) was approved by the US Food and Drug Administiration for the palliative treatment of mycosis fungoides since 1988. Herein, we present our MF patients who have received ECP.
Patients and Methods: We retrospectively included 50 MF patients who have diagnosed at our center. ECP was given empirically in cycles of 2 consecutive days in every 2 to 4 weeks at any time during their follow-up.
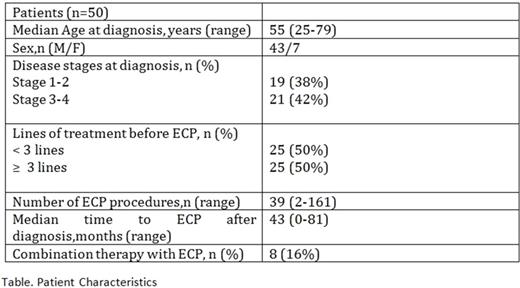
Results: The patient characteristics is shown in table. Previous lines of treatments were; topical retinoids (bexarotene), topical corticosteroids, phototherapy (PUVA), Narrowband ultraviolet B light (NBUVB), Interferons, Metotrexate (MTX), CHOP (cyclophosphamide, daunorubicin, vincristine, prednisolone). ECP is combined with gemcitabine, PUVA, MTX, Bexaroten, IFN or Vorinostat. The overall response rate (ORR) was 54% with 30% complete response rate (CRR). 10/15 complete responders (67%) had stage 3-4 disease at diagnosis and 7/15 (47%) had received ≥ 3 lines of treatment prior to ECP. 18 patients (36%) had progressive disease after ECP while 3 patients (6%) were refractory and underwent allogeneic stem cell transplantation. The few adverse events of ECP included in 8 patients (16%) as catheter-related infection, headache, fever, chills and nausea. The OS was 68 months.
Conclusion: ECP is a favorable treatment option for suitable patients in MF with high ORR and low risk of adverse events.
Presenter has relevant financial relationship(s) to disclose: No.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal