Abstract
Introduction:
As per the 2008 World Health Organization (WHO) classification, Myeloproliferative neoplasms (MPN) are subclassified into eight clinicopathological groups.1 The discovery of activating JAK2 mutations revolutionized the approach to diagnosis of MPN. Recently there have been studies suggesting an increasing number of mutations distinct from JAK2 associated with MPN. The new mutations being studied are MPL with a mutation frequency of 1-5% commonly seen in Essential Thrombocytosis (ET) and Primary Myelofibrosis (PMF).2 IDH1 has a mutational frequency of 21% for blast phase of MPN and 4% for PMF.3 Other mutations that have been found to be coexistent with MPNs are EZH2, TP53 and TET2.4
Methods:
A total of 79 cases with clinical suspicion of MPN were studied over one year. Based on WHO criteria, a total of 20 cases were diagnosed as MPN taking into account complete blood counts, bone marrow and cytogenetic studies with molecular profiling using next generation sequencing (NGS). Out of these, 13 cases were diagnosed as JAK2 positive and 7 as JAK2 negative MPN. All cases were studied for a panel of 50 mutations (Table 1) using NGS (Ion Torrent PGM) and a minimum coverage of 100x was considered to be significant.
Mutation Panel
| ABL1 . | EGFR . | GNAQ . | KRAS . | PTPN11 . |
|---|---|---|---|---|
| AKT1 | ERBB2 | GNAS | MET | RB11 |
| ALK | ERBB4 | HNF1A | MLH1 | RET |
| APC | EZH2 | HRAS | MPL | SMAD4 |
| ATM | FBXW7 | IDH1 | NOTCH1 | SMARCB1 |
| BRAF | FGFR1 | IDH2 | NPM1 | SMO |
| CDH1 | FGFR2 | JAK2 | NRAS | SRC |
| CDKN2A | FGFR3 | JAK3 | PDGFRA | STK11 |
| CSF1R | FLT3 | KDR | PIK3CA | TP53 |
| CTNNB1 | GNA11 | KIT | PTEN | VHL |
| ABL1 . | EGFR . | GNAQ . | KRAS . | PTPN11 . |
|---|---|---|---|---|
| AKT1 | ERBB2 | GNAS | MET | RB11 |
| ALK | ERBB4 | HNF1A | MLH1 | RET |
| APC | EZH2 | HRAS | MPL | SMAD4 |
| ATM | FBXW7 | IDH1 | NOTCH1 | SMARCB1 |
| BRAF | FGFR1 | IDH2 | NPM1 | SMO |
| CDH1 | FGFR2 | JAK2 | NRAS | SRC |
| CDKN2A | FGFR3 | JAK3 | PDGFRA | STK11 |
| CSF1R | FLT3 | KDR | PIK3CA | TP53 |
| CTNNB1 | GNA11 | KIT | PTEN | VHL |
Results:
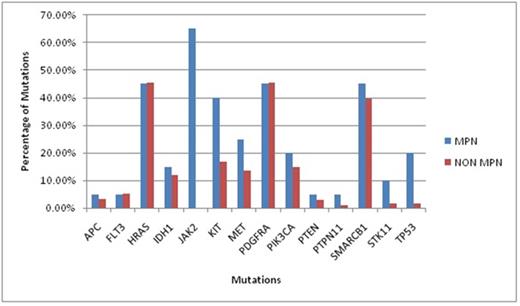
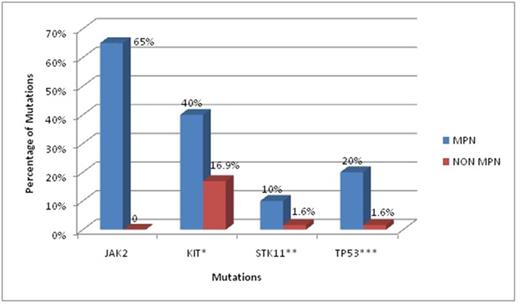
Out of 20 MPN cases there were 3 cases of Polycythemia Vera (PV) 8 cases of ET and 9 cases of PMF. From the 14 mutations found in MPN, JAK 2 (65%) was the commonest followed by HRAS (45%), PDGFRA (45%), SMARCB1 (45%), Kit (40%), MET (25%), TP53 (20%), PIK3CA(20%), IDH1 (15%), STK11 (10%), APC (5%), FLT3(5%),PTEN (5%) and PTPN11(5%) (Graph1). Apart from JAK2, statistically significant mutations in the MPN group as compared to the non MPN group were Kit, TP53 and STK11 (Graph 2). Kit showed Single Nucleotide Polymorphism (SNP) with substitution of Adenosine (A) with Cytosine(C) on chr4:55593464 (hg19) and was statistically significant in MPN group as compared to non MPN group (40 % vs16.9 %, p value = 0.016). TP53 mutation showed a C to A SNP on chr17:7577036 (hg19) which was found more often in MPN groups than non MPN (20 % vs. 1.6 %, p value = 0.0018). STK11 mutation showed a C to Guanine (G) SNP on chr19:1223125 and a G insertion at chr19:1221320 and was found to be more associated with MPN than non MPN (10% vs. 1.6%, p value = 0.046) . However all other mutations were statistically insignificant.
Graph 1: Total Mutations in MPN versus Non MPN
Graph 2: Significant mutations in MPN
*p-value: 0.016, **p-value: 0.046, ***p-value: 0.0018
In the MPN group, 13 cases were JAK2 positive and 7 cases were JAK2 negative. There was no statistical significance of presence of mutations between the two groups. In our study there was no significant association of IDH1 in MPN group in comparison to non MPN group ( 15% vs. 11.86%, p value = 0.35), in contrast to earlier studies.3
Conclusion:
The mutations in Kit, TP53 and STK11 were found to be significantly more in cases of MPN as compared to non MPN. In future studies, different mutations present in MPN should be identified using NGS which will be crucial to not only diagnose and further characterize MPN cases but also for better understanding of the stepwise pathogenesis leading to cancer development in humans and to develop new targeted therapies. This is the first study of its kind in Indian population, to the best of our knowledge.
References: 1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoetic and Lymphoid Tissues.
4th ed. Lyon: International Agency for Research on Cancer (IARC); 2008. 2. Akpinar TS, Hancer VS, Nalcaci M, Diz-Kucukkaya R. MPL W515L/K Mutations in Chronic Myeloproliferative Neoplasms.Turk J Haematol. 2013 March; 30(1): 8-12.
3. Tefferi A, Lasho TL, Abdel-Wahab O, Guglielmelli P, Patel J, Caramazza D, et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia . 2010. July ;24(7):1302-9.
4. Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I,et al., Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014 Apr 3;123(14):2220-8.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal