Abstract
Flow cytometric analysis of the surface antigen profile of bone marrow (BM) cells has been shown to increase diagnostic sensitivity when combined with BM morphology and conventional cytogenetics (CG) and is currently recommended (Porwit et al Leukemia 2014). We wanted to test the differential diagnostic value of multiparameter flow cytometry (MFC) in a tertiary referral hospital with a wider array of colors to detect distinct aberrancies in antigen expression using simultaneously altogether 10 antibody conjugates.
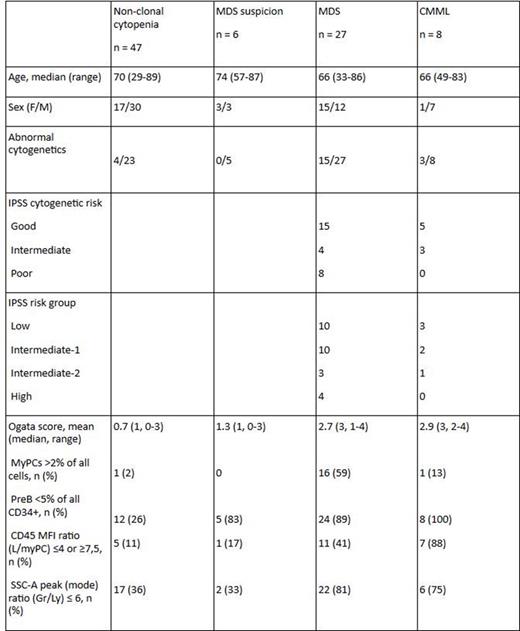
From I/2013 to VII/2015, BM samples from 88 pts with unclear cytopenia or suspected MDS were analyzed in parallel by cytomorphology (CM), CG and MFC. Anemia and/or thrombocytopenia were typical findings at presentation, median age 69 years (range: 29-89), M/F ratio 1.44:1. They were followed until end-VII/2015, for a median time of 15 months (1-30). The 35 MDS or CMML diagnoses were based on the WHO 2008 classification, distribution: 1 RA, 2 RT, 2 RARS, 10 RCMD, 2 RAEB-1, 5 RAEB-2, 2 MDS-U, 3 del(5q), 7 CMML-1 and 1 CMML-2. The two other groups: pts in whom MDS could not be confirmed during the follow-up (MDS suspicion) and those who were diagnosed as various non-clonal cytopenias.
MFC was performed with 10-color flow in 4-5 tubes following ELN recommendations (Westers et al Leukemia 2012) in myeloid progenitor cells (myPC), granulocytes and monocytes. As a modification to the ELN protocol, the novel AML stem cell-associated antigen, C-type Lectin-like molecule-1 (CLL-1, also termed ClecA12, van Rhenen et al Blood 2007) together with CD38 was studied on myPCs. Our analysis included the robust four parameter flow cytometric score (FCM score) originally reported by Della Porta and ELN (Haematologica 2012). In their study, a FCM score ≥2 was significantly associated with MDS.
The value of the recently published strategy to identify patients with a deletion 5q on the basis of MFC (Oelschlaegel et al Haematologica 2015) was also evaluated for patients recruited since I/2014 (n = 50). Parameters included in the 5q- score: 1) CD45 MFI ratio (Ly/myPC) ≤7.0 - score 10, 2) myPC >2% - score 3, 3) SSC-ratio (Gr/Ly) <6 -score 2, 4) CD71 on Gr ≤20% - score 1.5, 5) Sex, female - score 1.5.
As MFC controls we had BM samples from six healthy subjects.
Clinical, cytogenetic and flow cytometric characteristics of the study cohort:
In myPCs, defined as CD34+, CD117+ cells, the most common aberrant lymphoid markers expressed were CD7, CD5 and CD56 (5, 4 and 2 of 35 MDS/CMML patients, respectively). Abnormal CLL-1 expression was seen in four of 35 MDS/CMML patients (in 3 pts it was expressed on CD38neg/dim myPCs and in one it was negative on all myPCs). In five patients, percentage of myPCs expressing CLL-1 was higher compared with normal controls: two of those pts had MDS, two non-clonal cytopenias and one was in the MDS suspicion group. A 5-parameter-del(5q)-score was studied in 50 patients, 20 with MDS/CMML and 30 with non-clonal cytopenia. Four patients had 5-parameter-del(5q)-score ≥15,0, all in the MDS group. Of those, two had deletion 5q, one t(3;3)(q21;q26) and monosomy 7, one had a complex karyotype.
In MDS diagnostics, 10-color flow cytometry allows better characterization of small blast populations and analysis of a wider selection of antigens in fewer tubes compared with conventional 4-5 color flow. It also provides a rough scoring system to help to screen potential MDS patients among unclear cytopenias. We found CD7, CD5 and the novel AML stem cell-associated antigen CLL-1 to be the most commonly aberrantly expressed antigens on myPC in MDS/CMML patients studied. This study also confirmed the value of the Ogata score as a screening tool in differential diagnostics of unclear cytopenias. With the del(5q)-score, we were able to identify both two patients with deletion 5q in 50 patients evaluated so far.
Ebeling:Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal