Abstract
International prognostic scoring system (IPSS) has been employed for prognosis assessment of myelodysplastic syndrome (MDS). However, clinical course of IPSS-intermediate-1 (Int1) MDS is heterogeneous. Despite the introduction of Revised-IPSS (IPSS-R), clinical course and prognosis of intermediate-risk MDS patients are still controversial. Identification of the patients with poor prognosis among intermediate-risk MDS would be helpful for making treatment decision.
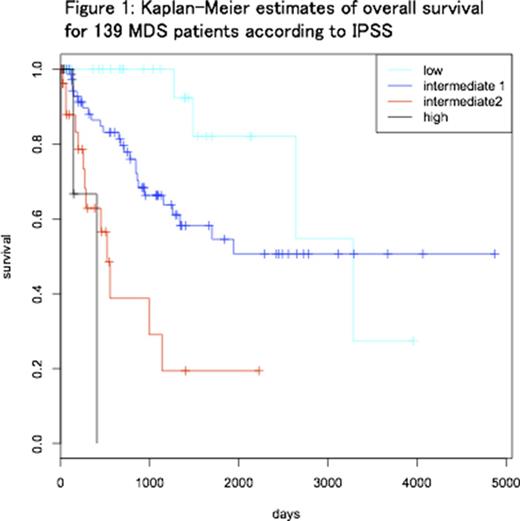
We retrospectively analyzed 139 patients (47 females and 92 males) who were diagnosed with MDS based on diagnostic bone marrow (BM) examination performed at our hospital. Median age at BM examination was 68 (18-88) years old. Patients were categorized as IPSS low (31), Int1 (74), Int2 (29) and high (5) (Figure 1). Based on IPSS-R, IPSS-Int1 MDS patients were subsequently divided into two groups: IPSS-RHigh (IPSS-R score ≥3.5; intermediate (28), high (7) and very high (2)) and IPSS-RLow (IPSS-R score ≤3; very low (1), low (36)). Median overall survival (OS) was significantly different (p = 0.006) between IPSS-RHigh (1154 days) and IPSS-RLow (not reached). AML-free survival was significantly longer in IPSS-RLow group (p=0.018).
To extract poorer-prognostic population from IPSS-RHigh patients, we analyzed serumlactate dehydrogenase (LDH) level, which is often elevated in patients with hematologic malignancies. Serum LDH data at the initial diagnosis was available for 36 out of 37 IPSSInt1/IPSS-RHigh MDS patients.
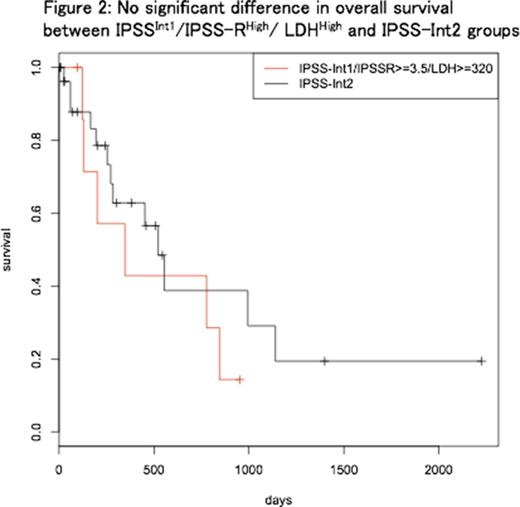
Patients with LDH ≥320 [U/L] (LDHHigh, n = 8) showed significantly shorter OS, compared with patients with LDH <320 [U/L] (n = 28) (median OS 347 and 1339 days, respectively; p=0.03). Between IPSSInt1/IPSS-RHigh/ LDHHigh (n=8) and IPSS-Int2 (n=29) groups, there was no significant difference regarding OS (p=0.41) (Figure 2) and AML-free survival (p=0.34). Median OS was 347 and 522 days; two-year cumulative incidence of AML was 0.42 and 0.25, respectively.
Recent studies using next-generation sequencing revealed genomic profiling of MDS. To understand the mutational pattern of IPSS-Int1 MDS, targeted deep sequencing using next generation sequencer, Ion Proton (Life Technologies), was performed for 26 IPSS-Int1 MDS patients with available genomic DNA samples, following written informed consent. We examined mutations in 11 commonly-mutated genes, TET2, SF3B1, ASXL1, SRSF2, DNMT3A, RUNX1, U2AF1, ZRSR2, STAG2, TP53 and EZH2. In total, 12 gene mutations were observed; the average number of mutations per patient was 0.46. SF3B1 mutation was most frequent (n=6), followed by TP53, DNMT3A, EZH2 and RUNX1 mutations. A total of 6 SF3B1 mutations including K700E (n=4), K666T (n=1) and R625C (n=1) were detected in 5 patients; one patient harbored two different SF3B1 mutations (K700E and K666T). WHO criteria for 5 patients with SF3B1 mutation included 4 RCUD (refractory cytopenia of unilineage dysplasia) and 1 RCMD (refractory cytopenia with multilineage dysplasia). None of the patients with SF3B1 mutation died or progressed into AML during the observation, while AML progression rate was 24% among the patients without SF3B1 mutation.
Although IPSS Int1 has been regarded as low-risk category, our results confirmed its heterogeneity. Sub-classification of IPSS-Int1 MDS patients using IPSS-R revealed that IPSS-RHigh patients showed shorter OS and AML-free survival compared with IPSS-RLow patients, however the prognosis among IPSSInt1/IPSS-RHigh patients was still heterogeneous. From IPSSInt1/IPSS-RHigh patients, serum LDH level successfully extracted higher-risk patients, whose prognosis was no better than that of IPSS-Int2 MDS patients.
Mutational status has not been considered either in IPSS or in IPSS-R. Our result suggests that SF3B1 mutation indicates better prognosis without blast increase or AML progression. On the other hand, none of 26 IPSS-Int1 MDS patients harbored TET2 mutation, the most frequently mutated gene among MDS patients. TET2 mutation have been shown to often present with higher BM blasts percentage. In our analysis, only 4 out of 26 subjects presented BM blasts above 5%, which might explain the absence of TET2 mutation.
Combination of IPSS-R and serum LDH is beneficial to extract patients with poorer prognosis from IPSS-Int1 MDS patients. In the near future, gene mutation profiles including SF3B1 can be a useful marker for risk stratification among heterogeneous IPSS-Int1 MDS patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal