Abstract
Bortezomib is a very useful drug in the treatment of multiple myeloma (MM) patients. Used in combination with other antineoplastic drugs it has a well documented impact in progression free survival (PFS) and overall survival (OS) of any age patients, elegible or not for hematopoietic cell transplant. Standard dose (1,3 mg/m2) is used in almost all patients and low dose (0,7-0,8mg/m2) is reserved to patients with kidney disease and neuropathy.
However, bortezomib doses used in phase 1 and 2 initial studies were described between 0,7 and 1,3 mg/m2 and were equally effective.
The aim of this study was to evaluate the cytometric response of MM naive patients to two different bortezomib doses.
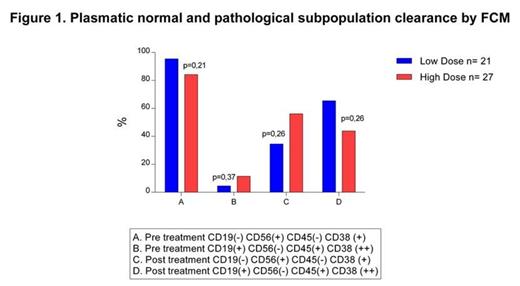
We retrospectively analyzed the flow cytometry of fourty eight patients with naive MM treated with VCD scheme (Bortezomib-Cyclophosphamide-Dexametasone), without kidney failure nor neuropathy, of whom 21 received low doses of bortezomib (0.8mg/m2) and 27 standard doses (1.3 mg/m2). Flow cytometry was analyzed at diagnosis and at the end of treatment according to the expression of several clusters of differentiation (CD), establishing the presence of plasmoblastic myeloma clones (>95% of plasma cells with immunophenotype CD19 (-) CD56(+) CD45(-) CD38(+) and restriction of intra cytoplasmatic kappa and or lambda light chains) and normal mature plasma cells (CD19(+) CD56(-) CD45 (+) CD38 (++) with a policlonal expression of kappa/lambda light chains). Cytometric complete response was defined as normalization of the immunophenotype of plasma cells and absence of pathological cells.
We found no statistical differences between the 2 groups in flow cytometric response (p>0.1), as shown in figure 1.
This retrospective analysis suggests that lower doses of bortezomib could have similar effects in disease control, at least in cytometric response, to standard doses. Further studies should be made to evaluate clinical response and overall survival in patients treated with low doses compared to standard doses of bortezomib. In our country high costs of new generation antineoplastic drugs makes it necessary to find less expensive and equally effective schemes in order to make these well known beneficial treatments available to a greater number of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal