Abstract
Background: Iron overload in patients receiving red blood cell (RBC) transfusions for treatment of anemia and in patients with non-transfusion-dependent thalassemia (NTDT) can lead to impaired organ function and is associated with significant morbidity and mortality. Regular iron load monitoring is essential and iron chelation therapy (ICT) should be promptly initiated when appropriate. Serum ferritin (SF) >1000 ng/mL is commonly used as an indicator of adverse clinical outcome resulting from iron overload. However, this is an indirect measure of tissue iron, whereas MRI allows accurate, reproducible assessment of cardiac and hepatic iron load, and its use may lead to improved patient care. The TIMES study used MRI to assess prevalence and severity of cardiac and hepatic siderosis in a large population of Australian patients with transfusion-dependent anemia or NTDT.
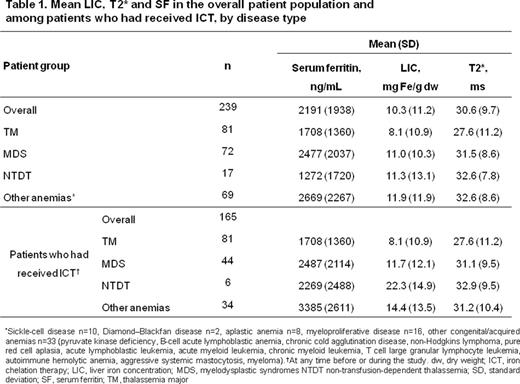
Methods: TIMES was an epidemiological study to assess cardiac and hepatic iron load in Australian, adult patients with thalassemia major (TM), NTDT (β thalassemia intermedia, β thalassemia/Hb E, Hb H disease), myelodysplastic syndromes (MDS) or other chronic anemias. Patients with NTDT had SF>300 ng/mL; others had a lifetime history of ≥20 units RBC transfusions and SF>500 ng/mL. Past medical history was collected (including anemia, RBC transfusion, ICT and hematologic data). Prospective MRI (FerriScan) was used to determine R2 liver iron concentration (LIC; siderosis >5 mg Fe/g dw for NTDT, >7 mg Fe/g dw for others) and myocardial T2* (siderosis <20 ms). The percentage of patients with hepatic and/or cardiac siderosis and mean LIC, SF and T2* were calculated for each disease group and according to ICT status. Linear regression was performed between screening SF and LIC within 60 days.
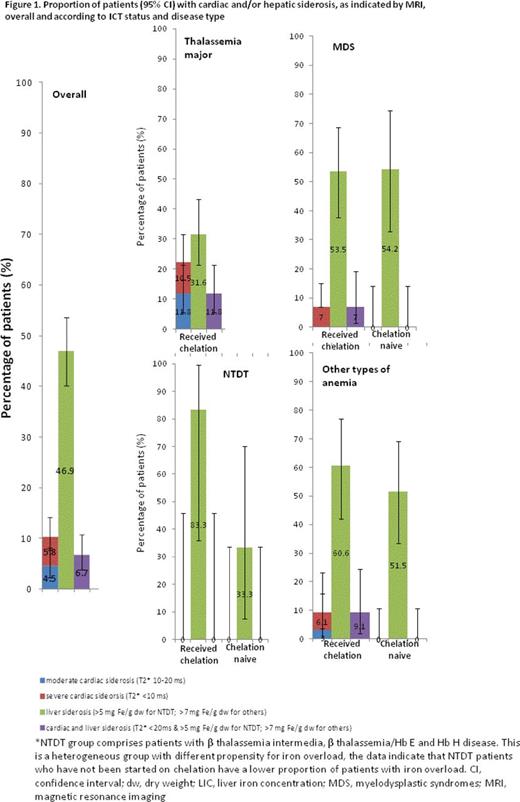
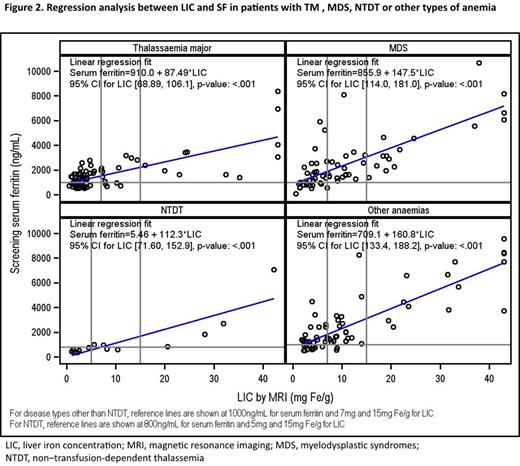
Results: 243 patients were enrolled and data for 239 were available for this analysis; 56% were male, 82% Caucasian, 10% Asian, 0.4% Aboriginal/Torres Strait Islander and 7% were of other ethnicity. Median age was 49.0 years (range 18-92). 77% of patients received ICT at any time before or during the study (59.8% deferasirox, 14.2% deferoxamine, 2.5% deferiprone), although not necessarily during the study. The degree of iron overload as determined by MRI is shown in Fig 1 and Table 1. Cardiac and hepatic siderosis was seen in 10 and 47% of patients, respectively. The prevalence of cardiac and hepatic siderosis in the different disease groups was: TM (22 and 32%), MDS (5 and 54%), NTDT (0 and 53%), and other anemia types (5 and 56%). All patients with cardiac siderosis had received ICT. Among patients with MDS, NTDT or other anemia types, some patients had iron overload (hepatic siderosis) but had not received ICT (MDS 19.4, NTDT 20.0, other anemias 25.8%). In all disease groups, mean LIC was above the target range (3-7 mg Fe/g dw, as established for TM by risk of morbidity), while mean cardiac T2* was above 20 ms. Although correlation between LIC and SF is observed in each disease group, some patients with high LIC had relatively low SF, indicating that SF is not an accurate indicator of iron load in these patients (Fig 2). Among patients analyzed (n=220), few (3.4%) patients with TM, MDS or other anemia types with SF<1000 ng/mL had LIC ≥7 mg Fe/g dw, confirming the utility of this SF chelation target in clinical practice; while in NTDT, 2 of 15 patients had LIC ≥5 mg Fe/g dw despite SF<800 ng/mL, supporting the importance of MRI evaluation in this subgroup.
Conclusion: In a large population of patients with heterogeneous causes of chronic anemia and elevated SF, there was a high prevalence of hepatic siderosis in all disease groups (32% in TM patients, who all received ICT; >50% in other disease groups), and a high prevalence of cardiac siderosis in TM patients (22%) despite ICT. Treatment guidelines recommend ICT initiation in patients with iron overload, followed by adequate dose adjustment and duration of ICT to maintain SF<1000 ng/mL, and to decrease risk of iron excess-related morbidities. Despite these recommendations, this study suggests iron overload still occurs in a large proportion of susceptible patients. Furthermore, not all patients in need of ICT can be identified using SF; MRI assessment of iron load is preferable. These data provide a real-life assessment of the magnitude of iron load, emphasizing the importance of adequate monitoring in patients with anemias receiving RBC transfusions and those with NTDT, to enable timely initiation of ICT and ongoing dose optimization.
Ho:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Aspen: Other: Conference Grant. Hiwase:Celgene Corporation: Research Funding. Viiala:Novartis: Honoraria. Zor:Novartis: Employment. Gervasio:Novartis: Employment. Bowden:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal