Abstract
Introduction: Up to 30% of patients (pts) undergoing stem cell mobilization with granulocyte colony-stimulating factor (G-CSF) with or without prior chemotherapy mobilize poorly. Plerixafor is a potent CXCR4 receptor antagonist that is licensed for stem cell mobilization in autologous stem cell donors with multiple myeloma (MM) or lymphoma, who show poor mobilization after stimulation with recombinant G-CSF (r-metHuG-CSF, Filgastrim). Here we present our experience with plerixafor in poorly mobilizing autologous donors.
Patients and Methods: We performed 35 stem cell collections between January 2011 and December 2014 in 28 poorly mobilizing autologous donors with MM (n=14), non-Hodgkin lymphoma (NHL, n=11), Hodgkin lymphoma (n=1) or both MM & NHL (n=2), adding plerixafor to the stimulation regimen with recombinant G-CSF. Known risk factors for poor mobilization included: diagnosis of lymphoma (50%); older age (61% >60y at apheresis); >1 chemotherapy lines (86%) & pretreatment with melphalan (18%). Median age of patients (pts) was 61 years (y, range 37-72y) at stem cell collection and 15 of the 28 pts were females. All pts received previous treatment with G-CSF (2x5µg/kg body weight [BW]) followed by a large volume leukapheresis (maximal 4 x total blood volume). All pts received 0.24 mg/kg BW plerixafor following insufficient mobilization of CD34+ cells into the peripheral blood (PB, <10/µl CD34+ cells) - either after peripheral reconstitution of white blood cells (WBC, >1 Gpt/l) following chemotherapy (n=22, 79%) or after 5 days of stimulation with G-CSF in steady state (n=6, 21%). Six pts treated with chemotherapy prior to mobilization received a second dose of plerixafor in response to insufficient CD34+ cell harvesting on the first collection day.
As a control cohort, we evaluated the data of 151 collections from MM, NHL or Hodgkin lymphoma pts (106 males, 45 females, median age 56y, range 22-74y) with efficient mobilization in response to G-CSF treatment over the same time period.
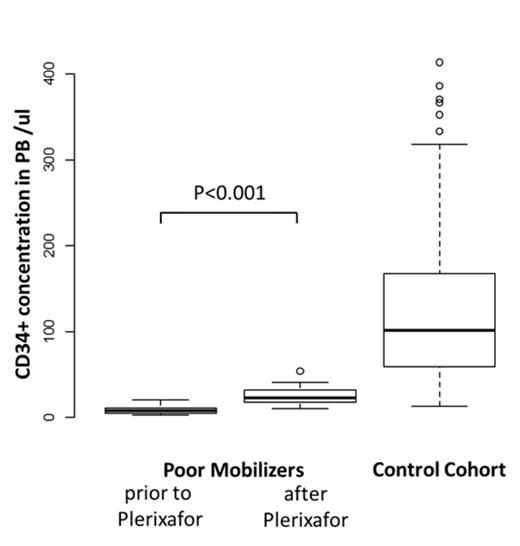
Results: The median concentration of CD34+ cells in PB before & after the first application of plerixafor was 8.4/µl (range 2.9-20.5 /µl) & 23.1/µl (range 10.2-54.1 /µl) respectively, corresponding to a median 3.3-fold increase (range 1.4-5.3-fold, P<0.001, Figure 1). In comparison, pts in the control group had a median concentration of 101.9/µl (range 13-1190/µl) CD34+ cells in PB on the day of stem cell collection (Figure 1).
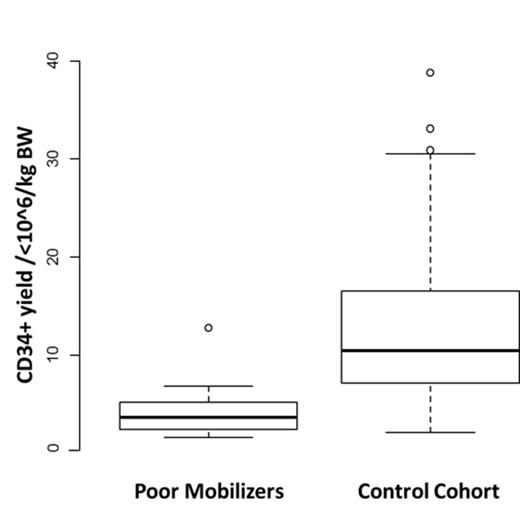
The median CD34+ cell yield for pts receiving plerixafor was 3.7x10^6/kg BW (range 1.6-12.8x10^6/kg BW) compared to 10.5x10^6/kg BW (range 2.1-38.8x10^6/kg BW) in the control cohort (Figure 2).
As expected, the WBC count in the product was higher in the plerixafor group (median 899.9x10^8, range 446-2214x10^8) compared to the efficiently mobilizing control cohort (median 470.2x10^8, range 99.9 -1537.1x10^8, P<0.001). The vitality of the cells after thawing was tested with methan blue staining & was comparable in both groups: median 85.3% (range 73.3-92.8%) in the plerixafor group & 84.4% (range 50.6-96.8%) in the control cohort (P=0.242). Furthermore, all harvests were tested for their proliferation capacity by quantifying colony forming units (CFU). Pts receiving plerixafor had a median CFU number of 22.5 (range 1-199), which did not differ from the control cohort with a median of 200.2 CFUs (range 1-1341, P=0.568). To date, 22 (78.6%) of the poorly mobilizing pts have received an autologous stem cell transplantation. All showed good peripheral WBC (median day 11, range 10-15) & platelet (median day 16, range 11-46) recovery.
Discussion: Our data shows that addition of plerixafor can increase the efficacy of stem cell harvesting in poor mobilizers. The collected stem cells fulfilled the appropriate quality control criteria including vitality after thawing (>50%) & proliferation capacity. We identified a median 3.3-fold increase in CD34+ cell concentration in PB & a median CD34+ cell yield of 3.7x10^6/kg BW after the application of plerixafor in poor mobilizers. Despite the fact that the CD34+ cell yield in pts receiving plerixafor remained significantly lower than the control cohort, we were able to harvest >2x10^6/kg BW CD34+ cells in 93% (n=26) of poor mobilizers. Thus, the mobilization outcome of pts receiving plerixafor is acceptable and within the expected range.
Franke:BMS: Honoraria; MSD: Other: Travel Costs; Novartis: Other: Travel Costs. Niederwieser:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal