Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is the only effective, even curative treatment for refractory/relapsed AML patients. Unmanipulated haploidentical HCT (haplo-HCT) resulted in encouraging outcomes for treatment of hematologic malignancies and become an alternative option in case of lacking HLA matched sibling donor (MSD). Unmanipulated haplo-HCT from G-CSF mobilized bone marrow and peripheral blood stem cell (PBSC) has shown similar results as that from MSD-HCT in leukemia. Here, we report the results of a cohort study on the efficacy and toxicity of haplo-PBSCT compared with MSD-PBSCT for treatment of refractory/relapsed AML.
PATIENTS AND METHODS
Among 419 newly diagnosed AML patients, 69 patients relapsed during CR1 and were planned to receive allo-HCT after re-induction. The order of preference of donors was MSD, matched unrelated (HLA 10/10 or 9/10 loci matched), or haploidentical donor. Thirty patients received haplo-PBSCT and 13 patients MSD-HCT (July, 2007 ~ June, 2014) at our unit. There was no difference of the characteristics of demography, disease or transplantation between these two groups (Table 1).
High-resolution DNA techniques were used to evaluate the HLA-A, B, DRB1, DQB1, and C loci. Donors were treated with rhG-CSF (5 mg.kg-1.day-1) for consecutive days. The PBSCs were collected on day 5 - 6 and infused on the day of collection.
The conditioning regimen consisted of Bu (9.6 mg.kg-1, intravenously, days -10 ~ -8), Carmustine, (250 mg.m-2, day -5), cytarabine (8 g.m-2, days -7 ~ -6), CY (120 mg kg-1, days -4 ~ -3), and ATG (rabbit; 10 mg.kg-1, days -5 ~ -2). MSD-HCT patients had the same conditioning regimen without ATG. All transplant recipients received cyclosporine A, mycophenolate mofetil, and short-term methotrexate for GVHD prophylaxis. The endpoint of the last follow-up for all surviving patients was June 30, 2015.
RESULTS
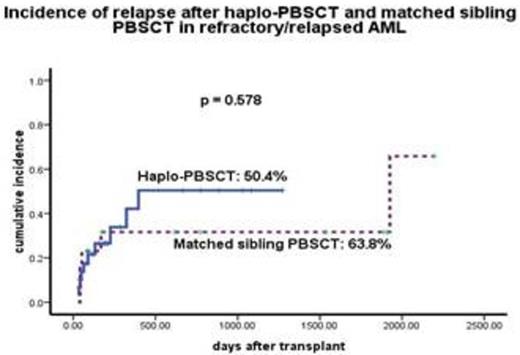
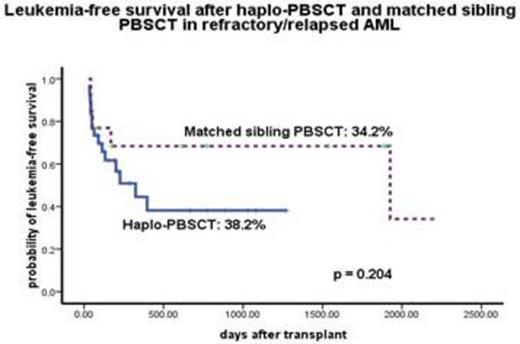
Sustained myeloid engraftment with full donor chimerism was achieved in both groups (100%) at a median of 16 (10 - 26) days. Twenty-six patients (86.7%) in haplo-PBSCT group and all patients in MSD-PBSCT group achieved platelet recovery. There was no difference of the cumulative incidence of acute GVHD grade 2-4 (Fig. 1), chronic GVHD (20% vs 33.3%, P=0.581), transplantation-related mortality (TRM) (16.7% vs 0%, P = 0.121), relapse (33.3% vs 38.5%, P = 0.578, Fig 2) between haplo-PBSCT and MSD-PBSCT group. Donor age of 41yr and older was an independent risk factor for inferior leukemia-free-survival (27.8% vs 37.2%, P = 0.004).
CONCLUSION
In this cohort study, haplo-PBSCT showed similar outcomes in patients with refractory/relapsed AML compared with MSD-PBSCT. It suggested the feasibility of G-CSF-primed PBSC as a graft source in unmanipulated haplo-HCT under myeloablative conditioning, which was effective and tolerable for treatment of poor risk leukemia.
Characteristics of patients and donors
| . | Haploidentical donor . | Matched sibling donor . | P value . | ||
|---|---|---|---|---|---|
| Cases . | % . | Cases . | % . | ||
| Gender, n (%) | |||||
| Receipt | |||||
| Male | 22 | 73.3 | 8 | 61.5 | 0.485 |
| Donor | |||||
| Male | 22 | 73.3 | 7 | 53.8 | 0.292 |
| Age,y, median(range) | |||||
| Patient | |||||
| ≤40 y, n (%) | 21 | 70 | 6 | 46.2 | 0.178 |
| Donor | |||||
| ≤41 y, n (%) | 13 | 43.3 | 5 | 38.5 | 1.000 |
| AML, n (%) | 1.000 | ||||
| CR2 | 5 | 16.7 | 2 | 15.4 | |
| NR/beyond CR2 | 25 | 83.3 | 11 | 84.6 | |
| Time to transp | 0.51 | ||||
| ≥7m | 14 | 46.7 | 8 | 61.5 | |
| Conditioning Regimen, n (%) | 0.675 | ||||
| BuCy | 22 | 73.2 | 9 | 69.2 | |
| TBIcy | 4 | 13.3 | 1 | 7.7 | |
| FB | 4 | 13.3 | 3 | 23.1 | |
| CD34+ in graft (106/kg) | 0.499 | ||||
| ≥4.77 | 17 | 56.7 | 5 | 41.7 | |
| . | Haploidentical donor . | Matched sibling donor . | P value . | ||
|---|---|---|---|---|---|
| Cases . | % . | Cases . | % . | ||
| Gender, n (%) | |||||
| Receipt | |||||
| Male | 22 | 73.3 | 8 | 61.5 | 0.485 |
| Donor | |||||
| Male | 22 | 73.3 | 7 | 53.8 | 0.292 |
| Age,y, median(range) | |||||
| Patient | |||||
| ≤40 y, n (%) | 21 | 70 | 6 | 46.2 | 0.178 |
| Donor | |||||
| ≤41 y, n (%) | 13 | 43.3 | 5 | 38.5 | 1.000 |
| AML, n (%) | 1.000 | ||||
| CR2 | 5 | 16.7 | 2 | 15.4 | |
| NR/beyond CR2 | 25 | 83.3 | 11 | 84.6 | |
| Time to transp | 0.51 | ||||
| ≥7m | 14 | 46.7 | 8 | 61.5 | |
| Conditioning Regimen, n (%) | 0.675 | ||||
| BuCy | 22 | 73.2 | 9 | 69.2 | |
| TBIcy | 4 | 13.3 | 1 | 7.7 | |
| FB | 4 | 13.3 | 3 | 23.1 | |
| CD34+ in graft (106/kg) | 0.499 | ||||
| ≥4.77 | 17 | 56.7 | 5 | 41.7 | |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal