Abstract
Background: A single genomic event is sufficient to cause CML; Ph translocation and the resulting BCR-ABL fusion. Additional genomic lesions accompany progression, which occurs very rapidly after diagnosis (dx) in a minority. Identification at dx of patients (pts) with poor prognosis remains an important goal and new sequencing technology enhances the prospects of uncovering pathologically relevant lesions for early warning of disease progression.
Aim: To determine the somatic genomic landscape at dx, the risk conferred by genomic lesions towards blast crisis (BC), and whether mechanisms that underlie CML progression are shared by other malignancies.
Method: Sequencing the whole exome (WES) and transcriptome (RNAseq) of paired tumor-normal samples (bone marrow mesenchymal stromal cells or remission) identified somatic single nucleotide variants, indels and gene fusions. Twenty-eight chronic phase (CP) first line imatinib (IM) treated pts were tested: 14 had BC at a median of 9 mo, r 3-60; and 14 had good response (MMR by 6 mo). Also tested were 4 pts diagnosed in advanced phase (2 accelerated phase [AP]; 2 BC) and 5 historical pts with BC at a median of 64 mo, r 38-100.
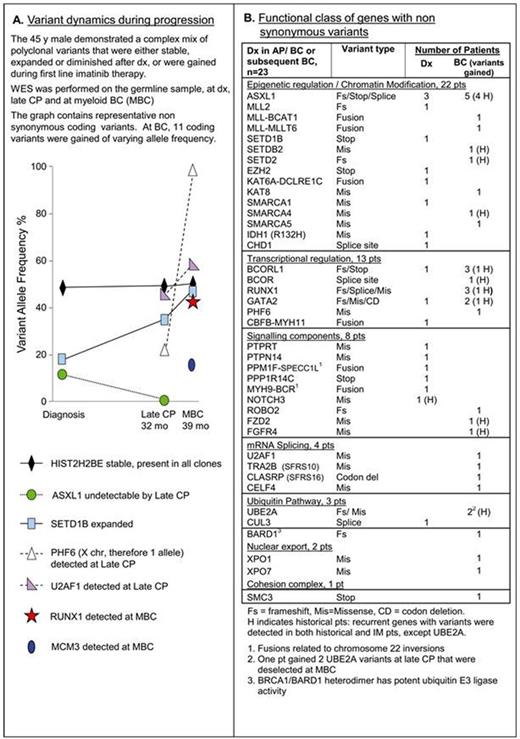
Results: At dx, a median of 33 somatic variants were detected per pt (r 1-62). The number of variants did not correlate with response, or CP vs AP/BC, but increased with age (r =0.48, P =.007), consistent with accumulation of variants in stem cells with aging and suggesting that many may be "passenger mutations". Non synonymous protein coding variants were present at a median of 7 per pt at dx (r 0-17), again without difference between groups. Most variants had an allele frequency close to 50%, indicating their likely presence in all leukemic cells. However, polyclonality at dx was evident by variants with low allele frequency that either expanded or diminished at BC, Fig A.
All 4 pts in AP/BC at dx had mutations in genes implicated in cancer pathogenesis (cancer genes) at dx; CBFB-MYH11 fusion, BCORL1, GATA2 and PTPRT, and SMARCA1. Of the 28 pts with first line IM, 11 had 15 somatic and 1 germline non synonymous variants/fusions at dx of known/potential significance: oncogenic mutations in IDH1 (R132H) and TP53 (germline R248Q); 6 frameshift/stop/splice site mutations in ASXL1, 1 EZH2 stop, 1 SETD1B stop, 1 MLL2 frameshift, 1 CHD1 splice site; and 4 novel fusions. Two of the fusions were generated by inversions of 2-13 MB of chr 22: PPM1F-SPECC1L (truncating the protein phosphatase PPM1F) and MYH9-BCR (truncating MYH9, reported to regulate p53 stability). Of these 11 pts, 9 had BC at a median of 6 mo of IM, r 3-39, and 2 had MMR by 6 mo. The 2 good response pts had ASXL1 mutations (both stop) and 1 also had a fusion involving chr 9 and 22 (TNRC6B-NEK6). The frequency of BC in CP pts with potentially pathogenic variants at dx was significantly higher than pts without such variants; 9/11 (82%) vs 5/17 (29%), P =.02.
At BC, 18 pts had WES performed. A median of 6 non synonymous variants were gained (r 0-15) including 1-4 mutations in cancer genes in 15/18 pts, Fig B. Six of 13 first line IM pts also had 11 BCR-ABL KD mutations at BC (8 P loop) and 5/6 were among the pts who acquired mutations in cancer genes. The pt with the germline oncogenic TP53 mutation acquired a novel ANKRD11-UBQLN1 fusion at BC at 5 mo. Interestingly, ANKRD11 is a key regulator of the oncogenic potential of this mutation.
In total, 6 genes were recurrently mutated; ASXL1, BCORL1, RUNX1, GATA2, MLL and UBE2A. Mutations occurred in genes that primarily belonged to classes mutated in AML, Fig B. Of the 23 AP/BC samples, 22 had non synonymous variants in genes involved in epigenetic regulation/chromatin modification. Variants were also detected in genes involved in ubiquitination and nuclear export, including an XPO1 variant reported in CLL. Nucleocytoplasmic transport has been implicated in IM resistance.
Conclusion: Risk of BC was significantly associated with cancer gene mutation or novel fusions at dx. Some mutated pathways in CML were common to other cancers. Epigenetic regulation/chromatin modification appears to play a central role in CML pathogenesis, and ubiquitination and nuclear export may be of emerging relevance. Notably, most pts with BCR-ABL KD mutations at BC also acquired cancer associated mutations, indicating multiple mechanisms may contribute to progression. Future testing at dx will likely include tumor/normal exome/transcriptome sequencing to aid risk stratification.
Branford:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Research Funding; Qiagen: Membership on an entity's Board of Directors or advisory committees. Yeung:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hughes:ARIAD: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal