Abstract
Introduction. MRD in ALL is defined as detection of leukemic cells in bone marrow by polymerase chain reaction (PCR) or flow cytometry with hematologic complete remission (CR). Pts with persistent/recurrent MRD after first-line induction therapy have a higher risk of relapse than those with complete MRD response (no detectable MRD with minimum sensitivity 0.01%). Interventions, including hematopoietic stem cell transplantation (HSCT), are used to improve the outcome of these pts. Blinatumomab, a bispecific T cell engager (BiTE®) antibody construct, redirects CD3+ T cells to CD19+ target cells, resulting in serial lysis of CD19+ B cells. In a multicenter, international phase 2 study in MRD+ ALL (Goekbuget N et al. Blood 2014;124:379), blinatumomab resulted in complete MRD response in cycle 1 in 78% of pts including multiple subgroups such as pts in second-line treatment, those with high MRD burden, and older pts. No subgroups with higher MRD complete response rates were identified. This analysis evaluated long-term outcomes, including overall survival (OS), relapse-free survival (RFS), and duration of remission (DOR).
Methods. Adults (≥18 years) with B-cell precursor ALL with hematologic CR (<5% blasts in bone marrow) and MRD ≥10-3 after ≥3 intensive chemotherapy treatments were eligible. Pts with CNS pathology or extramedullary disease, previous allogeneic HSCT, or Philadelphia-chromosome positive (Ph+) ALL eligible for tyrosine kinase inhibitors were excluded. Blinatumomab 15 µg/m²/day was given by continuous IV infusion for 4 weeks, followed by a 2-week break (1 cycle). MRD was measured by a central laboratory using PCR per EuroMRD guidelines. MRD responders in cycle 1 received up to 3 additional cycles or underwent HSCT. Pts with hematologic relapse discontinued treatment. We report here preliminary follow-up data as of 1 July 2015. Final data from the preplanned 18-mo follow-up analysis will be available for the meeting.
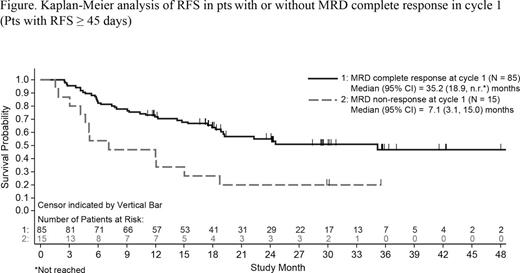
Results. 116 pts enrolled and received blinatumomab. Median age was 45 years (range 18-76); 15 (13%) pts were age ≥65 years. 90 (78%) pts received HSCT after blinatumomab. 62 (53%) pts were still being followed. 35 pts relapsed and 26 pts died in CR (23 of them after subsequent HSCT). Median OS, with median follow-up of 29.5 mo, was 36.5 mo (95% CI, 19.1 mo to not reached [n.r.]): 40.4 vs 12.0 (P =.001) in pts with (n=88) or without (n=24) MRD complete response in cycle 1. 110 pts were evaluable (CR at study entry, Ph-) for RFS and DOR. Median RFS was 18.9 mo (95% CI, 12.3 to 35.2 mo): 24.6 vs 11.0 (P =0.005) in pts treated in first (n=75) vs later (n=35) remission; and 35.2 vs 7.1 (P =0.002) in pts alive and relapse-free after 45 days with (n=85) or without (n=15) MRD complete response in cycle 1 (Figure). Median DOR was n.r. (95% CI, 24.6 mo to n.r.): n.r. vs 15.0 mo (P =0.002) in pts treated in first vs later remission; and n.r. vs 15.0 mo (P =0.015) in pts with DOR ≥ 45 days with (n=85) or without (n=13) MRD complete response in cycle 1. In time-dependent Cox model analyses, HSCT vs no HSCT were not different for OS (hazard ratio [HR], 1.39; 95% CI, 0.68 to 2.82; P =0.368) or RFS (HR, 0.89; 95% CI, 0.47 to 1.69; P =0.730); DOR (treating death as a competing risk) was longer for HSCT vs no HSCT (HR, 0.36; 95% CI, 0.17 to 0.77; P =0.008). All pts experienced at least one AE. The most clinically relevant were neurologic events, including tremor (30%), aphasia (13%) dizziness (8%), ataxia and paraesthesia (6% each), and encephalopathy (5%). Rates decreased over time (cycles 1, 2, 3, and 4) for any neurologic event (47%, 24%, 15%, and 15%) and any grade ≥3 neurologic event (10%, 4%, 0%, and 0%). 12 (10%) pts interrupted treatment due to grade ≥3 neurologic events: 5 resumed without another interruption and 2 resumed then stopped treatment for another neurologic event. Investigators reported 4 deaths as fatal AEs during follow-up (brain injury, disease progression, gastrointestinal hemorrhage, and multiorgan failure); all 4 pts received HSCT after blinatumomab.
Conclusion. In this long-term follow-up analysis of the first large prospective trial with an experimental compound in MRD+ ALL, MRD complete response induced by single-agent blinatumomab treatment was associated with longer OS, RFS, and DOR compared with not achieving an MRD complete response after blinatumomab treatment. This strengthens the current strategy of MRD-based treatment in ALL before occurrence of clinical relapse.
Gökbuget:Sanofi: Equity Ownership; Pfizer: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Erytech: Consultancy; Eusapharma/Jazz: Consultancy, Honoraria, Research Funding; Gilead Sciences: Consultancy; Kite: Consultancy; Medac: Consultancy, Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria, Research Funding; Bayer: Equity Ownership; SigmaTau: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria. Off Label Use: Blinatumomab (BLINCYTO®) is approved for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). It has not been approved for use in patients in hematologic remission, but with presence of minimal residual disease (MRD), from ALL.. Dombret:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Bonifacio:Amgen: Consultancy; Ariad Pharmaceuticals: Consultancy; Pfizer: Consultancy; Novartis Farma: Research Funding. Reichle:University Hospital Regensburg: Employment. Graux:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Faul:Amgen: Honoraria. Topp:Astra: Consultancy; Regeneron: Consultancy; Affimed: Consultancy, Research Funding; Roche: Consultancy, Other: Travel Support; Jazz: Consultancy; Pfizer: Consultancy; Amgen: Consultancy, Honoraria, Other: Travel Support. Brüggemann:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria. Horst:Pfizer: Research Funding; MSD: Research Funding; Gilead: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Boehringer Ingleheim: Research Funding. Stieglmaier:Amgen Research (Munich) GmbH: Employment; Amgen Inc: Equity Ownership. Wessels:Amgen: Employment. Haddad:Amgen Ltd.: Employment; Amgen Inc.: Equity Ownership. Zugmaier:Amgen Res. Munich: Employment. Nagorsen:Amgen: Employment, Equity Ownership, Patents & Royalties: Inventor on blinatumomab-related patent. Bargou:University of Wuerzburg, Germany: Employment; Novartis: Consultancy, Honoraria; GEMoaB GmbH: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Patents & Royalties: Patent for blinatumomab.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal