Abstract
Congenital macrothrombocytopenia (CMTP) is a heterogeneous group of disorders characterized by abnormally large platelets and low platelet counts. So far, genes responsible for CMTP have been identified in approximately half of the cases. The genes currently known are MYH9, GP1BA, GP1BB, GP9, ITGA2B, ITGB3, ACTN1 and other less frequent genes. However, in the remaining half, CMTP-causing genes are unknown. To identify genes causing CMTP, we first performed whole exome sequencing and target sequencing in 46 Japanese CMTP families and identified 1 family with a heterozygous mutation in GFI1B gene. Several groups have recently reported GFI1B as a causative gene for CMTP with alpha-granule deficiency and red cell anisopoikilocytosis. Here, we report on the identification of a novel mutation in GFI1B gene and its functional characterization.
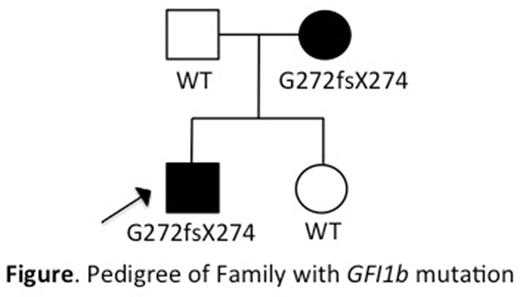
We performed whole exome sequencing and additional targeted sequencing in genetically undefined 46 CMTP families and identified 1 family with GFI1B variant (Figure), which co-segregated with CMTP. The proband (arrow in the Figure) of this family was a 15 years old boy with a history of easy bruising and platelet count of 70-115x109/L. His mother also had low platelet count, although details of her bleeding complications are unknown. Morphologic analysis of peripheral blood smear from the affected family members showed gray appeared enlarged platelets and red cell anisocytosis. Subsequent sequencing analysis confirmed a heterozygous point mutation (c.G814+1C, p.G272fsX274) at the splice donor site flanking exon 6 of GFI1B. GFI1B contains six zinc fingers (Znfs), of which Znf 3 to 5 are essential for DNA binding. The mutated transcript predicted a truncation of 58 C-terminal amino acids, which resulted in complete deletion of Znf 5. Since previous reports on GFI1B mutations have also described mutations of Znf 5 (p.H294fsX307 and p.Q287X mutations by Stevenson et al and Monteferrario et al, respectively), we performed biological analyses of these 3 patient-derived mutations affecting essential DNA-binding domain.
GFI1B is an essential transcriptional factor for megakaryocyte and erythroid development. Therefore, we tested the effects of the GFI1b mutations on the transcriptional repression of firefly luciferase reporter constructs containing GFI1B promoter as a validated GFI1B target. Whereas wild-type GFI1B repressed the expression of reporter gene, the G272fsX274, H294fsX307 and Q287X mutants were unable to repress the expression. To test whether these patient-derived mutations acted in dominant-negative manner, we measured the effect of increasing the amount of mutant GFI1B cDNA, cotransfected into cells with a set amount of wild-type GFI1B cDNA. All 3 mutants inhibited gene repression mediated by wild-type GFI1B in a dose-dependent manner.
To investigate biological effects of mutant GFI1B on proplatelet formation from megakaryocytes, we expressed the mutant GFI1B in mouse fetal liver cells (FLCs) by retrovirus-mediated gene transfer. FLCs harvested from embryonic day 13.5 embryos were transfected with pGCDNsamIRES/EGFP vector containing wild-type or the 3 patient-derived mutant GFI1B and were cultured with thrombopoietin. And we observed proplatelet formation of EGFP positive megakaryocytes. The number of proplatelet-producing megakaryocytes was reduced in mutant GFI1B transduced FLCs on day 3 and 4 after transfection, suggesting that mutant GFI1B inhibited the rate of proplatelet production from megakaryocytes. Next, we investigated the size and number of proplatelet tips from megakaryocytes, and found that proplatelet tips produced from G272fsX274, H294fsX307 and Q287X-transduced megakaryocytes were significantly reduced in numbers, but were increased in the size of the tips. This was compatible with decreased platelet count and enlarged platelet seen in GFI1B mutated patients. These findings indicated that patient-derived mutant GFI1B disrupted proplatelet formation of megakaryocyte.
In summary, we have identified a novel GFI1B mutation that causes autosomal dominant macrothrombocytopenia with gray platelets and red cell anisocytosis. The mutant GFI1B acted in a dominant-negative manner over wild-type GFI1B. In addition, we first showed that the mutant-transduced FLCs produced abnormally enlarged proplatelet tips, which was reduced in numbers.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal