Abstract
Background: Blood products, supportive care, and hypomethylating agents (HMAs) are frequently used to improve outcomes of patients with MDS, and they may incur substantial costs. It is unclear whether disease-related costs of care are associated with OS in MDS patients. We evaluated the relationship between MDS-specific costs and survival among Medicare-enrolled beneficiaries with MDS in the US.
Methods: The study cohort consisted of patients aged ≥66 years who were diagnosed with MDS (International Classification of Diseases for Oncology, 3rd edition, codes: 9980, 9982, 9983, 9985-7, 9989) between 1/1/2005 and 12/31/2011 in the linked Surveillance, Epidemiology, and End Results (SEER) - Medicare database. Only patients with continuous enrollment in Medicare Parts A and B from one year before MDS diagnosis through death or end of study follow up (12/31/2012) were included. OS was calculated from date of diagnosis to date of death, and patients alive at the end of the study were censored. Medicare payments were used to estimate costs and adjusted to 2012 US dollars. Cumulative costs in a matched group of cancer-free Medicare beneficiaries were subtracted from costs in the MDS cohort in each of the 12 SEER states to estimate MDS-related costs for each state. Comorbidities within one year before diagnosis were identified and used to calculate a modified Elixhauser comorbidity score and a disability status score (a proxy measure of performance status). We used 2-year OS for primary analysis as it was the major endpoint in several clinical trials evaluating MDS therapies. States were separated into 3 tertiles according to 2-year MDS-related costs per patient. Kaplan-Meier methods were used to compare OS probabilities at various time points, stratified by MDS-related cost groups (3 levels). Cox proportional hazard regression models were used to assess the impact of MDS-related costs (3 levels) on survival, controlling for age at diagnosis, sex, race, comorbidities, disability status, pre-diagnosis cost, median household income at the zip code level, "ever" use of HMA, and MDS histologic subtype. We also performed a sensitivity analysis involving patients who did not use any HMAs (HMA non-user subcohort) and separate analyses using 3-year MDS-related costs.
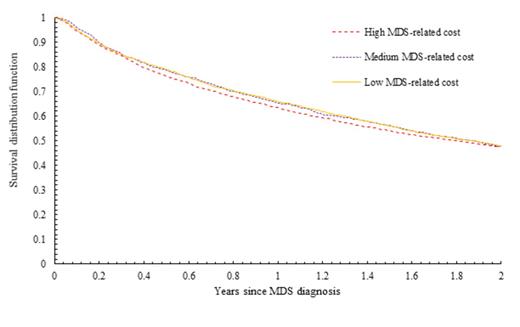
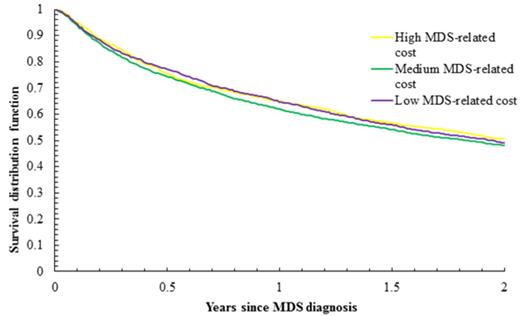
Results: Of 24,347 patients diagnosed with MDS, 8,564 met eligibility criteria. Of those, 86.7% were white, 53.0% males, 52.5% ≥80 years at diagnosis, and 15.7% received hypomethylating agents (HMAs). By end of follow-up, 6,011 patients (70.2%) had died. Median follow-up was 1.57 years for all patients and 3.17 years for living patients. The 2-year OS was 48.7% and the median OS was 1.84 years. The median 2-year MDS-related cost of care per patient was $67,717 (California), and it ranged between $43,950 and $83,961 across 12 states. As expected, the costs were higher among HMA-users (Range: $109,447 - 156,156) than non-users (Range: $36,250 - 55,446). In a multivariate model of the entire study cohort, factors associated with improved survival included female gender, non-white race, younger age at diagnosis, refractory anemia and refractory anemia with ring sideroblasts histologic subtypes, pre-diagnosis health costs, and lower Elixhauser comorbidity and lower disability status scores. The 2-year state-level MDS-related cost was not associated with MDS survival [reference: lowest tertile, hazard ratio (HR) for middle tertile was 1.02, 95% confidence interval (CI): 0.93-1.12, p = 0.74, and HR for the highest tertile was 0.99, 95% CI: 0.92-1.06, p = 0.73] (Figure 1). Among the HMAs non-users (n=7,222), there was also no association between the 2-year MDS-related costs and MDS survival (Figure 2). When we conducted separate analyses using 3-year MDS-related costs, we observed no association between costs and survival in the overall study cohort or in the HMA non-user subcohort.
Conclusions: Medicare expenditures for elderly patients with MDS varied substantially across states, but were not associated with overall survival. The lack of association between costs and outcome warrants additional research, as it may help identify potential areas for cost saving interventions without compromising outcomes.
Survival of MDS patients by two-year state-level MDS-related costs per patient (in 3 levels); p=0.50.
Survival of MDS patients by two-year state-level MDS-related costs per patient (in 3 levels); p=0.50.
Survival of MDS patients who did not use any HMAs, by two-year state-level MDS-related costs per patient (in 3 levels); p = 0.15.
Survival of MDS patients who did not use any HMAs, by two-year state-level MDS-related costs per patient (in 3 levels); p = 0.15.
Davidoff:Celgene: Consultancy, Research Funding. Gore:Celgene: Consultancy, Research Funding. Gross:21st-Century Oncology LLC: Research Funding; Johnson and Johnson: Research Funding; Medtronic: Research Funding. Ma:Incyte Corp: Consultancy; Celgene Corp: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal