Key Points
Low ADAMTS13 activity is associated with ischemic stroke.
ADAMTS13 activity improved the accuracy of ischemic stroke risk predictions beyond the traditional risk factors.
Abstract
ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin motif repeats 13) has antithrombotic properties because it cleaves von Willebrand factor (VWF) in smaller, less active multimers. The aim of our study was to investigate prospectively the association between ADAMTS13 activity and ischemic stroke. We included 5941 individuals ≥55 years without a history of stroke or transient ischemic attack (TIA) of the Rotterdam Study, a population-based cohort study. ADAMTS13 activity was measured at inclusion with the FRETS-VWF73 assay and VWF antigen (VWF:Ag) levels by enzyme-linked immunosorbent assay. We assessed the association among ADAMTS13 activity, VWF:Ag levels, and ischemic stroke by Cox proportional hazard analysis. The added value of ADAMTS13 activity above the traditional risk factors for ischemic stroke risk prediction was examined by the C-statistic and the net reclassification improvement index (NRI). All individuals were followed for incident stroke or TIA. Over a median follow-up time of 10.7 years (56 403 total person-years), 461 participants had a stroke, 306 of which were ischemic. After adjustment for cardiovascular risk factors, individuals with ADAMTS13 activity in the lowest quartile had a higher risk of ischemic stroke (absolute risk, 7.3%) than did those in the reference highest quartile (absolute risk, 3.8%; hazard ratio, 1.65; 95% confidence interval [CI], 1.16-2.32). Adding ADAMTS13 to the model in prediction of ischemic stroke, increased the C-statistic by 0.013 (P = .003) and provided 0.058 (95% CI, −0.002 to 0.119) NRI. Low ADAMTS13 activity is associated with the risk of ischemic stroke and improves the accuracy of risk predictions for ischemic stroke beyond traditional risk factors.
Introduction
Ischemic stroke is a major cause of mortality and morbidity in the Western world.1 Although many risk factors have been identified, its pathogenesis remains largely unclear and treatment options are still limited. The discovery of new risk factors might thus support the development of new preventive measures and treatment strategies.
A disintegrin and metalloproteinase with a thrombospondin motif repeats 13 (ADAMTS13), part of the ADAMTS family,2,3 has antithrombotic properties by cleaving ultralarge von Willebrand Factor (VWF) multimers into smaller forms.4 Ultralarge VWF multimers are the most procoagulant forms leading to platelet adhesion and aggregation and finally thrombus formation. Several studies have reported an association between high VWF levels and the risk of ischemic stroke.5-7 The importance of ADAMTS13 in circulation is exemplified by patients who develop thrombotic thrombocytopenic purpura (TTP) resulting from a severe deficiency of ADAMTS13, resulting in reduced cleavage of high-molecular-weight VWF multimers. This leads to an increase of these procoagulant VWF multimers, which in turn can result in microthrombus formation, frequently leading to disturbances of the cerebral circulation or other organs. In TTP patients, microthrombi may lead to focal neurologic deficit, seizures, and even coma.8 The importance of circulating ADAMTS13 was also shown in animal models. Several animal studies have indicated a pathogenic role of ADAMTS13 in the development or progression of ischemic stroke. Studies in which focal cerebral ischemia was experimentally induced showed larger cerebral infarctions in ADAMTS13-deficient mice than in wild-type mice.9-11
These data and small case-control studies suggest that ADAMTS13 plays a role in cardiovascular disease, but no prospective studies have yet established the relationship between the VWF cleavage protease ADAMTS13 and the risk of ischemic stroke (IS).7,12-15 To investigate the role of ADAMTS13 and VWF in the pathogenesis of ischemic stroke, we investigated prospectively the longitudinal association of ADAMTS13 activity, and its interaction with VWF and the risk of ischemic stroke in a large population-based cohort of nearly 6000 individuals above the age of 55. We also examined whether addition of ADAMTS13 activity to the traditional risk factors would lead to improvements in ischemic stroke risk prediction.
Material and methods
Study design and study population
We included participants from the Rotterdam Study (RS), a prospective population-based cohort study among individuals ≥55 years living in a suburb in the city of Rotterdam, The Netherlands.16,17 The study started in 1990 (RS-I). In 1999, additional individuals who had turned 55 years or had moved into the study district since the start of the study were added to the cohort (RS-II). All participants visited the research center every 3 to 4 years, where established cardiovascular risk factors are assessed. For this study, we used data obtained from participants at the third examination of the original cohort (RS-I-3, 1997-1999) and the first examination of the extended cohort (RS-II-1, 2000-2001). Protocols for the original and the extended cohort were similar. Previous findings of the Rotterdam Study are summarized in supplemental Table 6, available on the Blood Web site. The Rotterdam Study has been approved by the medical ethics committee according to the Population Study Act Rotterdam Study, executed by the Ministry of Health, Welfare and Sports of the Netherlands. Written informed consent was obtained from all participants.
Assessment of stroke and transient ischemic attack
History of stroke and transient ischemic attack (TIA) was determined during the baseline interview and verified in medical records. After enrollment in the Rotterdam Study, participants were continuously monitored for incident strokes and TIAs through automated linkage of the study database with files from general practitioners, the municipality, and nursing home physicians. Additional information was obtained from hospital records. Potential strokes were reviewed by research physicians and verified by an experienced stroke neurologist. Events were structured based on this information and a diagnosis was made based on the criteria independent of the diagnosis of the initial caregivers. Subarachnoid hemorrhages and retinal strokes were excluded. Strokes were classified as ischemic, hemorrhagic, or unspecified. Ischemic stroke was diagnosed if a computed tomography (CT) or magnetic resonance imaging (MRI) scan carried out within 4 weeks after the event ruled out other diagnoses. A hemorrhagic stroke was diagnosed if a relevant hemorrhage was shown on CT or MRI scan. If no neuroimaging was performed, the stroke was classified as unspecified. TIA was defined by focal symptoms that started suddenly and improved within seconds with a maximum duration of 24 hours.
Baseline characteristics
At inclusion in the study (RS-I-3 and RS-II-1), a detailed interview was taken from all participants, as well as an extensive set of examinations, including a physical examination and blood sampling. Blood pressure was calculated as the mean of 2 measurements using a random-zero sphygmomanometer at the right brachial artery while the individual was in a sitting position. Antihypertensive drugs were defined as the use of antihypertensive medication indicated for the treatment of high blood pressure (≥grade 1 hypertension according to World Health Organization criteria18 ). Grade 1 hypertension was defined as systolic >140 mm Hg or a diastolic blood pressure >90 mm Hg. Grade 2 hypertension was defined as systolic >160 mm Hg or a diastolic blood pressure >100 mm Hg. Antithrombotic medication was defined as the use of vitamin K antagonists, platelet aggregation inhibitors, and direct thrombin inhibitors. Diabetes mellitus was defined as the use of blood glucose–lowering medication and/or a fasting serum glucose level ≥7.0 mmol/L. Lipid-reducing agents were defined as the use of statins. Total cholesterol and high-density lipoprotein (HDL) cholesterol were measured using an automated enzymatic procedure. Body mass index (BMI) was calculated as the weight (in kilograms) divided by the square of the height (in meters). Smoking status was defined as current, former, and no smoking. Blood group antigen phenotypes were reconstructed by haplotypes analysis of 4 single nucleotide polymorphisms—rs687289, rs507666, rs8176704, and rs8176749—which collectively serve as tagging singe nucleotide polymorphisms for the O, A1, A2, and B alleles.
ADAMTS13 activity measurements
ADAMTS13 activity was measured in a kinetic assay using the fluorescence resonance energy transfer substrate VWF 73 (FRETS-VWF73) as previously described.19 Samples were measured against a reference curve of serial dilutions of normal human plasma defined to have an ADAMTS13 activity of 1 U/mL. Normal ADAMTS13 activity is 100%, with a range between 50% and 150%.20,21 Ten percent of the samples were retested and all were within 25% variation.
VWF antigen (VWF:Ag) levels were determined with an in-house enzyme-linked immunosorbent assay, using polyclonal rabbit anti-human VWF antibodies (DakoCytomation, Glostrup, Denmark) for catching and tagging.
Statistical analysis
Data on baseline characteristics are shown as mean and standard deviation (SD) for continuous variables and as counts and percentages for categorical variables. The association between ADAMTS13 and baseline characteristics was assessed by univariate and multivariable linear regression and presented as β-coefficients. β-coefficients represent the change in ADAMTS13 activity with an increase of 1 unit of the specific variable. In case of categorical variables, this means that having the characteristic is associated with a change of β in ADAMTS13 activity compared with participants who did not have the characteristic. To assess proportional hazards assumption, we tested the log minus log plots. The association between ADAMTS13 and stroke was performed by Cox proportional hazards regression analysis using ADAMTS13 quartiles, derived from the whole cohort. We examined the association of ADAMTS13 activity with all strokes (hemorrhagic, ischemic, and unspecified), ischemic strokes, TIA, and all cerebrovascular events (TIA and all strokes). Analyses with stroke were censored for TIA occurring before the stroke and analyses with TIA were censored for stroke. Consequently, participants were followed from inclusion to stroke, TIA, death, last health status update where they were known to be free of stroke or TIA, or January 1, 2012, whichever came first. Follow-up was complete until January 1, 2012 for 95.4% of potential person-years.22 The total number of person-years was 56 403. A curve with cumulative incidence of TIA and all strokes during follow-up was constructed for each of the ADAMTS13 activity quartiles. For the interaction between VWF:Ag and ADAMTS13 and the risk of stroke, we performed a Cox proportional hazard regression analysis with addition of an interaction term to the model. For the association between ADAMTS13 and VWF with the risk of stroke, a combination of ADAMTS13 activity ≤ and > the 25th percentile and VWF:Ag levels ≥ and < the 75th percentile were used. We adjusted all analyses for age and sex, and additionally for antithrombotic agents, antihypertensive drugs, diabetes mellitus, lipid-reducing agents, BMI, smoking, total cholesterol, HDL cholesterol, systolic blood pressure, and diastolic blood pressure.
To address the additional predictive value of ADAMTS13 in ischemic stroke risk prediction, we formed 2 models. The first model consisted of the variables included in the recent American Heart Association Pooled Cohort Equation: age, sex, systolic blood pressure, treatment of hypertension, total and HDL cholesterol, smoking, and diabetes.23 The second model additionally included quartiles of ADAMTS13 activity. We then compared the 2 models using the likelihood ratio χ2 test for global model fit, C-statistic for model discrimination, and net reclassification improvement index (NRI). Discrimination refers to the ability of the model to assign a higher risk to individuals who will develop an event compared with the individuals who will not. NRI specifies the amount of correct reclassification of individuals with event and individuals without event after extension of the model with ADAMTS13. The NRI estimates were based on the 10-year ischemic stroke risk categories of <5%, 5% to 7.5%, and >7.5%.24
Missing values of these covariates (0%-4.7%) were imputed 5 times using a multiple imputation method including all covariates. Statistical analyses were performed on all 5 different datasets and pooled into one final result using SPSS software. Data were analyzed with SPSS version 21 (SPSS 21.0. IBM, Somers, NY) and R, version 3.1.2. All statistical tests were 2-tailed and P values <.05 were considered statistically significant.
Results
The RS started with 7983 participants (of 10 215 invitees) and was extended with an additional 3011 participants (of 4472 invitees). We included all participants from whom blood was sampled (n = 6494). All participants with a history of stroke (n = 257) or TIA (n = 296) at the moment of blood sampling (1997-2001) were excluded from the analysis. A total of 5941 individuals >55 years of age were included in this study. Over a median follow-up time of 10.7 years (interquartile range, 7.9-11.6) and 56 403 total person-years, 461 individuals had a stroke, 306 of which (66%) were ischemic stroke, 48 (10%) that were hemorrhagic, and 107 (23%) classified as unspecified. A TIA occurred in 315 individuals during follow-up. The mean age of all individuals was 69 years (±8.1) and 57% were female (Table 1). The mean ADAMTS13 activity in the total cohort was 91.9 ± 17.8%. The mean time between ADAMTS13 activity measurement and the occurrence of stroke or TIA were 5.80 and 5.46 years, respectively.
Baseline characteristics of the total study population
| N = 5941 . | Total cohort, N (%) or mean ± SD . |
|---|---|
| Age (y) | 69.0 ± 8.1 |
| Female sex | 3401 (57.2%) |
| Smoking | |
| Current | 1020 (17.3%) |
| Former | 2907 (49.4%) |
| BMI (kg/m2) | 27.0 ± 4.1 |
| Use of antithrombotic medication | 952 (16.8%) |
| Use of antihypertensive drugs | 1314 (23.2%) |
| Use of lipid-reducing agents | 716 (12.6%) |
| Presence of diabetes mellitus | 599 (10.2%) |
| Total cholesterol (mg/dL)* | 224.7 ± 37.7 |
| HDL cholesterol (mg/dL)* | 53.8 ± 14.9 |
| Hypertension | |
| Systolic blood pressure (mm Hg) | 143 ± 21 |
| Diastolic blood pressure (mm Hg) | 77 ± 11 |
| Grade I hypertension | 2309 (39.1%) |
| Grade II hypertension | 879 (14.9%) |
| Blood group O | 2288 (45.3%) |
| ADAMTS13 activity (%) | 91.89 ± 17.8 |
| N = 5941 . | Total cohort, N (%) or mean ± SD . |
|---|---|
| Age (y) | 69.0 ± 8.1 |
| Female sex | 3401 (57.2%) |
| Smoking | |
| Current | 1020 (17.3%) |
| Former | 2907 (49.4%) |
| BMI (kg/m2) | 27.0 ± 4.1 |
| Use of antithrombotic medication | 952 (16.8%) |
| Use of antihypertensive drugs | 1314 (23.2%) |
| Use of lipid-reducing agents | 716 (12.6%) |
| Presence of diabetes mellitus | 599 (10.2%) |
| Total cholesterol (mg/dL)* | 224.7 ± 37.7 |
| HDL cholesterol (mg/dL)* | 53.8 ± 14.9 |
| Hypertension | |
| Systolic blood pressure (mm Hg) | 143 ± 21 |
| Diastolic blood pressure (mm Hg) | 77 ± 11 |
| Grade I hypertension | 2309 (39.1%) |
| Grade II hypertension | 879 (14.9%) |
| Blood group O | 2288 (45.3%) |
| ADAMTS13 activity (%) | 91.89 ± 17.8 |
SI conversion factors. To convert total cholesterol and HDL cholesterol to mmol/L, multiply values by 0.0259.
Baseline characteristics and ADAMTS13 activity
ADAMTS13 activity was associated with age and sex, also after adjustment for cardiovascular risk factors. In this cohort, we observed a decrease of 5.68% in ADAMTS13 activity per 10-year increase of age (β-coefficient, 5.68%; P < .001) and we found 8.6% higher ADAMTS13 activity in women vs men (P < .001; Table 2). Several cardiovascular risk factors were also associated with ADAMTS13 activity, including diabetes mellitus, cholesterol, and smoking (Table 2).
Association between ADAMTS13 and baseline characteristics
| . | Univariate β-coefficient (95% CI) . | P . | Multivariable β-coefficient (95% CI) . | P . |
|---|---|---|---|---|
| Every 10-y increase of age | −5.23 (−5.77 to −4.68) | <.001 | −5.68 (–6.28 to −5.09) | <.001 |
| Female sex | 7.19 (6.29 to 8.08) | <.001 | 8.60 (7.64 to 9.55) | <.001 |
| Antihypertensive drugs | 0.35 (–0.75 to 1.45) | .53 | −0.24 (–1.31 to 0.84) | .668 |
| Lipid-reducing agents | 1.62 (0.20 to 3.05) | .026 | 2.12 (0.69 to 3.54) | .004 |
| Antithrombotic medication | −4.52 (–5.76 to −3.28) | <.001 | −1.64 (–2.90 to −0.38) | .011 |
| Diabetes mellitus | 4.10 (2.59 to 5.61) | <.001 | 5.17 (3.72 to 6.62) | <.001 |
| Total cholesterol (mg/dL) | 0.05 (0.04 to 0.06) | <.001 | 0.03 (0.02 to 0.04) | <.001 |
| HDL cholesterol (mg/dL) | −0.02 (–0.05 to 0.02) | .315 | −0.12 (–0.15 to −0.08) | <.001 |
| Every 10 mm Hg increase in systolic blood pressure | −0.11 (–0.33 to 0.10) | .302 | 0.19 (–0.08 to 0.46) | .162 |
| Every 10 mm Hg increase in diastolic blood pressure | 0.57 (0.16 to 0.98) | .006 | −0.11 (–0.61 to 0.40) | .679 |
| BMI (kg/m2) | 0.30 (0.19 to 0.41) | <.001 | −0.04 (–0.15 to 0.07) | .472 |
| Current smoking | −3.17 (–4.37 to −1.98) | <.001 | −4.95 (–6.09 to −3.82) | <.001 |
| . | Univariate β-coefficient (95% CI) . | P . | Multivariable β-coefficient (95% CI) . | P . |
|---|---|---|---|---|
| Every 10-y increase of age | −5.23 (−5.77 to −4.68) | <.001 | −5.68 (–6.28 to −5.09) | <.001 |
| Female sex | 7.19 (6.29 to 8.08) | <.001 | 8.60 (7.64 to 9.55) | <.001 |
| Antihypertensive drugs | 0.35 (–0.75 to 1.45) | .53 | −0.24 (–1.31 to 0.84) | .668 |
| Lipid-reducing agents | 1.62 (0.20 to 3.05) | .026 | 2.12 (0.69 to 3.54) | .004 |
| Antithrombotic medication | −4.52 (–5.76 to −3.28) | <.001 | −1.64 (–2.90 to −0.38) | .011 |
| Diabetes mellitus | 4.10 (2.59 to 5.61) | <.001 | 5.17 (3.72 to 6.62) | <.001 |
| Total cholesterol (mg/dL) | 0.05 (0.04 to 0.06) | <.001 | 0.03 (0.02 to 0.04) | <.001 |
| HDL cholesterol (mg/dL) | −0.02 (–0.05 to 0.02) | .315 | −0.12 (–0.15 to −0.08) | <.001 |
| Every 10 mm Hg increase in systolic blood pressure | −0.11 (–0.33 to 0.10) | .302 | 0.19 (–0.08 to 0.46) | .162 |
| Every 10 mm Hg increase in diastolic blood pressure | 0.57 (0.16 to 0.98) | .006 | −0.11 (–0.61 to 0.40) | .679 |
| BMI (kg/m2) | 0.30 (0.19 to 0.41) | <.001 | −0.04 (–0.15 to 0.07) | .472 |
| Current smoking | −3.17 (–4.37 to −1.98) | <.001 | −4.95 (–6.09 to −3.82) | <.001 |
Univariate and multivariable linear regression analysis, β-coefficient represents the change in ADAMTS13 activity with 95% CI per unit increase of the selected variable.
ADAMTS13 activity and the risk of stroke
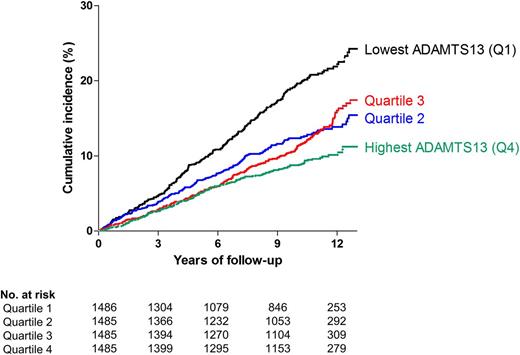
Compared with individuals in the highest quartile of ADAMTS13 activity, individuals with an ADAMTS13 activity within the lowest quartile had an increased risk of all strokes after adjustment for age and sex (absolute risk lowest quartile, 11.0%; highest quartile, 5.3%; hazard ratio [HR], 1.52; 95% confidence interval [CI], 1.15-2.02) and there was no statistically significant change after additional adjustment for cardiovascular risk factors (HR, 1.49; 95% CI, 1.12-2.00) (Table 3). There was no graded response effect from quartile 4 to quartile 1. Taking ADAMTS13 activity as a continuous variable (per SD decrease), we found an increased risk for stroke (HR, 1.12; 95% CI, 1.01-1.24) (Table 3). For ischemic stroke (absolute risk, 7.3%) the HR was 1.65 (95% CI, 1.16-2.32). The risk for ischemic stroke was also increased when taking ADAMTS13 as a continuous variable (HR, 1.19; 95% CI, 1.05-1.34). Individuals in the lowest quartile of ADAMTS13 activity also had an increased risk of TIA (absolute risk, 6.8%), and all cerebrovascular events (absolute risk, 17.8%) (HR, 1.64; 95% CI, 1.17-2.31 and HR, 1.56; 95% CI, 1.25-1.94, respectively). These results were similar for all outcomes if we adjusted additionally for prevalent coronary heart disease (supplemental Table 7) and for blood group. The cumulative incidence of TIA and all strokes per ADAMTS13 quartile over the total follow-up period is shown in Figure 1.
Cox proportional hazard regression analysis between ADAMTS13 quartiles and stroke
| ADAMTS13 level . | Mean ADAMTS13 activity (95% CI) . | Number of cases/Total number at risk . | Absolute risk (%) . | Model 1 HR (95% CI) . | Model 2 HR (95% CI) . |
|---|---|---|---|---|---|
| All strokes (N = 461) | |||||
| Quartile 1 | 70.3 (69.8-70.8) | 163/1486 | 11.0 | 1.52 (1.15-2.02) | 1.49 (1.12-2.00) |
| Quartile 2 | 86.3 (86.2-86.5) | 108/1485 | 7.2 | 1.10 (0.82-1.47) | 1.08 (0.80-1.45) |
| Quartile 3 | 96.6 (96.4-96.7) | 111/1485 | 7.5 | 1.18 (0.88-1.58) | 1.17 (0.88-1.57) |
| Quartile 4 | 114.4 (113.8-114.9) | 79/1485 | 5.3 | 1 (ref) | 1 (ref) |
| Per SD decrease | 1.13 (1.02-1.24) | 1.12 (1.01-1.24) | |||
| Ischemic strokes (N = 306) | |||||
| Quartile 1 | 70.3 (69.8-70.8) | 109/1486 | 7.3 | 1.61 (1.15-2.26) | 1.65 (1.16-2.32) |
| Quartile 2 | 86.3 (86.2-86.5) | 70/1485 | 4.7 | 1.07 (0.75-1.53) | 1.08 (0.75-1.54) |
| Quartile 3 | 96.6 (96.4-96.7) | 71/1485 | 4.8 | 1.12 (0.79-1.60) | 1.13 (0.80-1.62) |
| Quartile 4 | 114.4 (113.8-114.9) | 56/1485 | 3.8 | 1 (ref) | 1 (ref) |
| Per SD decrease | 1.18 (1.04-1.33) | 1.19 (1.05-1.34) | |||
| TIA (N = 315) | |||||
| Quartile 1 | 70.3 (69.8-70.8) | 101/1486 | 6.8 | 1.55 (1.11-2.18) | 1.64 (1.17-2.31) |
| Quartile 2 | 86.3 (86.2-86.5) | 78/1485 | 5.3 | 1.20 (0.85-1.69) | 1.25 (0.89-1.76) |
| Quartile 3 | 96.6 (96.4-96.7) | 77/1485 | 5.2 | 1.19 (0.84-1.67) | 1.21 (0.86-1.71) |
| Quartile 4 | 114.4 (113.8-114.9) | 59/1485 | 4.0 | 1 (ref) | 1 (ref) |
| Per SD decrease | 1.17 (1.03-1.24) | 1.19 (1.05-1.34) | |||
| Any cerebrovascular event (N = 776) | |||||
| Quartile 1 | 70.3 (69.8-70.8) | 264/1486 | 17.8 | 1.54 (1.24-1.91) | 1.56 (1.25-1.94) |
| Quartile 2 | 86.3 (86.2-86.5) | 186/1485 | 12.5 | 1.14 (0.91-1.42) | 1.15 (0.92-1.44) |
| Quartile 3 | 96.6 (96.4-96.7) | 188/1485 | 12.7 | 1.18 (0.95-1.48) | 1.19 (0.95-1.48) |
| Quartile 4 | 114.4 (113.8-114.9) | 138/1485 | 9.3 | 1 (ref) | 1 (ref) |
| Per SD decrease | 1.14 (1.06-1.23) | 1.15 (1.06-1.24) |
| ADAMTS13 level . | Mean ADAMTS13 activity (95% CI) . | Number of cases/Total number at risk . | Absolute risk (%) . | Model 1 HR (95% CI) . | Model 2 HR (95% CI) . |
|---|---|---|---|---|---|
| All strokes (N = 461) | |||||
| Quartile 1 | 70.3 (69.8-70.8) | 163/1486 | 11.0 | 1.52 (1.15-2.02) | 1.49 (1.12-2.00) |
| Quartile 2 | 86.3 (86.2-86.5) | 108/1485 | 7.2 | 1.10 (0.82-1.47) | 1.08 (0.80-1.45) |
| Quartile 3 | 96.6 (96.4-96.7) | 111/1485 | 7.5 | 1.18 (0.88-1.58) | 1.17 (0.88-1.57) |
| Quartile 4 | 114.4 (113.8-114.9) | 79/1485 | 5.3 | 1 (ref) | 1 (ref) |
| Per SD decrease | 1.13 (1.02-1.24) | 1.12 (1.01-1.24) | |||
| Ischemic strokes (N = 306) | |||||
| Quartile 1 | 70.3 (69.8-70.8) | 109/1486 | 7.3 | 1.61 (1.15-2.26) | 1.65 (1.16-2.32) |
| Quartile 2 | 86.3 (86.2-86.5) | 70/1485 | 4.7 | 1.07 (0.75-1.53) | 1.08 (0.75-1.54) |
| Quartile 3 | 96.6 (96.4-96.7) | 71/1485 | 4.8 | 1.12 (0.79-1.60) | 1.13 (0.80-1.62) |
| Quartile 4 | 114.4 (113.8-114.9) | 56/1485 | 3.8 | 1 (ref) | 1 (ref) |
| Per SD decrease | 1.18 (1.04-1.33) | 1.19 (1.05-1.34) | |||
| TIA (N = 315) | |||||
| Quartile 1 | 70.3 (69.8-70.8) | 101/1486 | 6.8 | 1.55 (1.11-2.18) | 1.64 (1.17-2.31) |
| Quartile 2 | 86.3 (86.2-86.5) | 78/1485 | 5.3 | 1.20 (0.85-1.69) | 1.25 (0.89-1.76) |
| Quartile 3 | 96.6 (96.4-96.7) | 77/1485 | 5.2 | 1.19 (0.84-1.67) | 1.21 (0.86-1.71) |
| Quartile 4 | 114.4 (113.8-114.9) | 59/1485 | 4.0 | 1 (ref) | 1 (ref) |
| Per SD decrease | 1.17 (1.03-1.24) | 1.19 (1.05-1.34) | |||
| Any cerebrovascular event (N = 776) | |||||
| Quartile 1 | 70.3 (69.8-70.8) | 264/1486 | 17.8 | 1.54 (1.24-1.91) | 1.56 (1.25-1.94) |
| Quartile 2 | 86.3 (86.2-86.5) | 186/1485 | 12.5 | 1.14 (0.91-1.42) | 1.15 (0.92-1.44) |
| Quartile 3 | 96.6 (96.4-96.7) | 188/1485 | 12.7 | 1.18 (0.95-1.48) | 1.19 (0.95-1.48) |
| Quartile 4 | 114.4 (113.8-114.9) | 138/1485 | 9.3 | 1 (ref) | 1 (ref) |
| Per SD decrease | 1.14 (1.06-1.23) | 1.15 (1.06-1.24) |
Model 1 adjusted for age and sex. Model 2 additionally adjusted for antithrombotic medication, antihypertensive drugs, diabetes mellitus, lipid-reducing agents, BMI, smoking, total cholesterol, HDL cholesterol, systolic blood pressure, and diastolic blood pressure. “All strokes” indicates ischemic, hemorrhagic, and unspecified strokes. Any cerebrovascular event indicates all strokes and TIA. Cutoff points (%) for quartiles were: ≤80.73%, 80.74-91.44%, 91.45-102.26%, and ≥102.27%.
Number of events per ADAMTS13 quartile. Number of all cerebrovascular events (TIA and all strokes) per quartile of ADAMTS13 activity. Cutoff points (%) of ADAMTS13 activity quartiles were: ≤80.73%, 80.74% to 91.44%, 91.45% to 102.26%, and ≥102.27%.
Number of events per ADAMTS13 quartile. Number of all cerebrovascular events (TIA and all strokes) per quartile of ADAMTS13 activity. Cutoff points (%) of ADAMTS13 activity quartiles were: ≤80.73%, 80.74% to 91.44%, 91.45% to 102.26%, and ≥102.27%.
ADAMTS13 activity and VWF:Ag levels
There was no relevant correlation between VWF:Ag levels and ADAMTS13 activity, although it was significant (R2 = 0.01, P < .001). Individuals who had both the lowest ADAMTS13 activity (≤25th percentile) and the highest VWF:Ag levels (≥75th percentile) had an increased risk of all strokes (absolute risk, 13.5%) and ischemic stroke (absolute risk, 9.1%) compared with the remaining individuals (HR, 1.49; 95% CI, 1.11-2.01 and HR, 1.71; 95% CI, 1.19-2.45, respectively) (Table 4). These data did not change when we additionally adjusted for prevalent coronary heart disease (supplemental Table 8). When comparing individuals with a low ADAMTS13 activity and high VWF:Ag with individuals with a high ADAMTS13 activity and low VWF:Ag as reference, this risk was even higher for all strokes (absolute risk, 13.5%) and ischemic stroke (absolute risk, 9.1%) (HR, 2.94; 95% CI, 1.49-5.78 and HR, 3.51; 95% CI, 1.60-7.70, respectively) (supplemental Table 9). Formal statistical testing did not reveal a significant interaction between VWF:Ag levels and ADAMTS13 activity in the association with all strokes, nor with ischemic stroke (P = .93 and P = .78, respectively).
Cox proportional hazard regression analysis among ADAMTS13 and VWF and stroke
| ADAMTS13 and VWF:Ag level . | Number of cases/Total number at risk . | Absolute risk (%) . | Model 1 HR (95% CI) . | Model 2 HR (95% CI) . |
|---|---|---|---|---|
| All strokes (N = 461) | ||||
| VWF < p75 and ADAMTS13 > p25 | 223/3395 | 6.6 | 1 (ref) | 1 (ref) |
| VWF ≥ p75 and ADAMTS13 > p25 | 76/1055 | 7.2 | 0.91 (0.70-1.19) | 0.92 (0.71-1.20) |
| VWF < p75 and ADAMTS13 ≤ p25 | 101/1032 | 9.8 | 1.24 (0.97-1.58) | 1.24 (0.97-1.60) |
| VWF ≥ p75 and ADAMTS13 ≤ p25 | 61/451 | 13.5 | 1.53 (1.14-2.06) | 1.49 (1.11-2.01) |
| Ischemic strokes (N = 306) | ||||
| VWF < p75 and ADAMTS13 > p25 | 153/3395 | 4.5 | 1 (ref) | 1 (ref) |
| VWF ≥ p75 and ADAMTS13 > p25 | 44/1055 | 4.2 | 0.83 (0.59-1.16) | 0.83 (0.59-1.17) |
| VWF < p75 and ADAMTS13 ≤ p25 | 68/1032 | 6.6 | 1.31 (0.98-1.76) | 1.34 (1.00-1.81) |
| VWF ≥ p75 and ADAMTS13 ≤ p25 | 41/451 | 9.1 | 1.72 (1.20-2.47) | 1.71 (1.19-2.45) |
| TIA (N = 315) | ||||
| VWF < p75 and ADAMTS13 > p25 | 156/3395 | 4.6 | 1 (ref) | 1 (ref) |
| VWF ≥ p75 and ADAMTS13 > p25 | 58/1055 | 5.5 | 1.06 (0.78-1.44) | 1.06 (0.78-1.44) |
| VWF < p75 and ADAMTS13 ≤ p25 | 72/1032 | 7.0 | 1.44 (1.08-1.92) | 1.49 (1.11-1.98) |
| VWF ≥ p75 and ADAMTS13 ≤ p25 | 29/451 | 6.4 | 1.27 (0.84-1.91) | 1.29 (0.85-1.95) |
| Any cerebrovascular event (N = 776) | ||||
| VWF < p75 and ADAMTS13 > p25 | 379/3395 | 11.2 | 1 (ref) | 1 (ref) |
| VWF ≥ p75 and ADAMTS13 > p25 | 134/1055 | 12.7 | 0.97 (0.80-1.19) | 0.97 (0.80-1.19) |
| VWF < p75 and ADAMTS13 ≤ p25 | 173/1032 | 16.8 | 1.32 (1.10-1.59) | 1.34 (1.11-1.61) |
| VWF ≥ p75 and ADAMTS13 ≤ p25 | 90/451 | 20.0 | 1.44 (1.13-1.83) | 1.43 (1.12-1.82) |
| ADAMTS13 and VWF:Ag level . | Number of cases/Total number at risk . | Absolute risk (%) . | Model 1 HR (95% CI) . | Model 2 HR (95% CI) . |
|---|---|---|---|---|
| All strokes (N = 461) | ||||
| VWF < p75 and ADAMTS13 > p25 | 223/3395 | 6.6 | 1 (ref) | 1 (ref) |
| VWF ≥ p75 and ADAMTS13 > p25 | 76/1055 | 7.2 | 0.91 (0.70-1.19) | 0.92 (0.71-1.20) |
| VWF < p75 and ADAMTS13 ≤ p25 | 101/1032 | 9.8 | 1.24 (0.97-1.58) | 1.24 (0.97-1.60) |
| VWF ≥ p75 and ADAMTS13 ≤ p25 | 61/451 | 13.5 | 1.53 (1.14-2.06) | 1.49 (1.11-2.01) |
| Ischemic strokes (N = 306) | ||||
| VWF < p75 and ADAMTS13 > p25 | 153/3395 | 4.5 | 1 (ref) | 1 (ref) |
| VWF ≥ p75 and ADAMTS13 > p25 | 44/1055 | 4.2 | 0.83 (0.59-1.16) | 0.83 (0.59-1.17) |
| VWF < p75 and ADAMTS13 ≤ p25 | 68/1032 | 6.6 | 1.31 (0.98-1.76) | 1.34 (1.00-1.81) |
| VWF ≥ p75 and ADAMTS13 ≤ p25 | 41/451 | 9.1 | 1.72 (1.20-2.47) | 1.71 (1.19-2.45) |
| TIA (N = 315) | ||||
| VWF < p75 and ADAMTS13 > p25 | 156/3395 | 4.6 | 1 (ref) | 1 (ref) |
| VWF ≥ p75 and ADAMTS13 > p25 | 58/1055 | 5.5 | 1.06 (0.78-1.44) | 1.06 (0.78-1.44) |
| VWF < p75 and ADAMTS13 ≤ p25 | 72/1032 | 7.0 | 1.44 (1.08-1.92) | 1.49 (1.11-1.98) |
| VWF ≥ p75 and ADAMTS13 ≤ p25 | 29/451 | 6.4 | 1.27 (0.84-1.91) | 1.29 (0.85-1.95) |
| Any cerebrovascular event (N = 776) | ||||
| VWF < p75 and ADAMTS13 > p25 | 379/3395 | 11.2 | 1 (ref) | 1 (ref) |
| VWF ≥ p75 and ADAMTS13 > p25 | 134/1055 | 12.7 | 0.97 (0.80-1.19) | 0.97 (0.80-1.19) |
| VWF < p75 and ADAMTS13 ≤ p25 | 173/1032 | 16.8 | 1.32 (1.10-1.59) | 1.34 (1.11-1.61) |
| VWF ≥ p75 and ADAMTS13 ≤ p25 | 90/451 | 20.0 | 1.44 (1.13-1.83) | 1.43 (1.12-1.82) |
Model 1 adjusted for age and sex. Model 2 additionally adjusted for antithrombotic medication, antihypertensive drugs, diabetes mellitus, lipid-reducing agents, BMI, smoking, total cholesterol, HDL cholesterol, systolic blood pressure, and diastolic blood pressure. “All strokes” indicates ischemic, hemorrhagic, and unspecified strokes. Any cerebrovascular event indicates all strokes and TIA. ADAMTS13 ≤25 percentile represents ≤80.72%, VWF ≥75th percentile ≥1.58 IU/mL.
Added predictive value of ADAMTS13 activity in ischemic stroke risk prediction
Table 5 summarizes the added value of ADAMTS13 for the prediction of ischemic stroke above the traditional risk factors. Addition of ADAMTS13 to the model, including the traditional risk factors, improved the model fit—that is, the likelihood ratio test statistics (χ2) increased from 123.7 to 136.9 (P = .013). The model including ADAMTS13 also increased the C-statistic by 0.013 (P = .003) in prediction of ischemic stroke. Addition of ADAMTS13 to the traditional risk factor model provided an NRI of 0.058% (95% CI −0.002 to 0.119) for the total population. Among subjects in the 5% to 7.5%, which can be considered an intermediate risk category for ischemic stroke, the NRI was 0.212 (95% CI, 0.048-0.376), composed of NRI of 13.5% for events and 7.7% for nonevents. Adding VWF to the model including traditional risk factors and ADAMTS13 did not provide any improvement in risk prediction.
Additional predictive information after extending the traditional risk factor model with ADAMTS13 in prediction of ischemic stroke
| . | . | Model fit . | Model discrimination . | |
|---|---|---|---|---|
| Models . | Likelihood ratio test statistics (χ2) . | C-statistic (95% CI) . | ||
| Traditional risk factor model | 123.7 | 0.694 (0.665, 0.723) | ||
| Traditional risk factor model + ADAMTS13 | 136.9* | 0.707 (0.678, 0.735)* | ||
| Reclassification after addition of ADAMTS13 to the traditional risk factors, total population (N = 5941) | ||||
| Subjects with event (%)† | Subjects without event (%)† | NRI (95% CI)‡ | ||
| Up | Down | Up | Down | |
| 15.0% | 9.4% | 8.8% | 9.0% | 0.058 (–0.002, 0.119) |
| Reclassification after addition of ADAMTS13 to the traditional risk factors, among the 5%-7.5% risk category (N = 1179) | ||||
| Subjects with event (%)† | Subjects without event (%)† | NRI (95% CI)‡ | ||
| Up | Down | Up | Down | |
| 33.3% | 19.8% | 20.3% | 28.0% | 0.212 (0.048, 0.376) |
| . | . | Model fit . | Model discrimination . | |
|---|---|---|---|---|
| Models . | Likelihood ratio test statistics (χ2) . | C-statistic (95% CI) . | ||
| Traditional risk factor model | 123.7 | 0.694 (0.665, 0.723) | ||
| Traditional risk factor model + ADAMTS13 | 136.9* | 0.707 (0.678, 0.735)* | ||
| Reclassification after addition of ADAMTS13 to the traditional risk factors, total population (N = 5941) | ||||
| Subjects with event (%)† | Subjects without event (%)† | NRI (95% CI)‡ | ||
| Up | Down | Up | Down | |
| 15.0% | 9.4% | 8.8% | 9.0% | 0.058 (–0.002, 0.119) |
| Reclassification after addition of ADAMTS13 to the traditional risk factors, among the 5%-7.5% risk category (N = 1179) | ||||
| Subjects with event (%)† | Subjects without event (%)† | NRI (95% CI)‡ | ||
| Up | Down | Up | Down | |
| 33.3% | 19.8% | 20.3% | 28.0% | 0.212 (0.048, 0.376) |
The traditional risk factor model is composed of the variables included in the American Heart Association Pooled Cohort Equation: age, sex, systolic blood pressure, treatment of hypertension, total and HDL cholesterol, smoking, and diabetes.
χ2, Chi-square statistic; CI, confidence interval; NRI, net reclassification improvement index.
P < .01 for the increase in model fit and for the increase in the C-statistic after addition of ADAMTS13 to the traditional risk factors.
Percentages of subjects with or without an event who move to a higher or lower risk category after adding ADAMTS13 to the traditional risk factors. The risk categories are based on 10-year ischemic stroke risk <5%, 5%-7.5%, and >7.5%.
NRI is estimated as ([number of events reclassified higher minus number of events reclassified lower]/number of events) + ([number of nonevents reclassified lower minus number of nonevents reclassified higher]/number of nonevents).
Discussion
In this prospective cohort study of nearly 6000 elderly individuals with a median follow-up of 10.7 years (56 403 total person-years), we observed an association between baseline ADAMTS13 activity and the development of ischemic stroke. Individuals with low ADAMTS13 activity had a higher risk of experiencing an ischemic stroke than individuals with high ADAMTS13 activity.
The association between ADAMTS13 levels and ischemic stroke has previously been investigated in small case-control studies.12-15 Although these studies also suggested that low ADAMTS13 levels are associated with an increased risk of stroke, they all had a cross-sectional design. Therefore, prospectively designed cohort studies, like the RS, are necessary to assess the role of ADAMTS13 as a risk factor for stroke.
The association between low ADAMTS13 activity and ischemic stroke risk is likely to be explained by less cleavage of high-molecular-weight multimers of VWF, which are the most prothrombotic forms. This results in more large procoagulant VWF multimers, and consequently a prothrombotic state, and may lead to thrombus formation at sites of endothelial damage and especially at sites with high shear stress, as in the arterial circulation. Nearly all individuals in the lowest ADAMTS13 quartile had ADAMTS13 activity in the normal range (50%-150%).20 Nonetheless, our study clearly suggests that moderately reduced ADAMTS13 activity is associated with an increased risk of thrombotic complications, even though the levels are not as low as what is characteristic for TTP patients. We did not find a graded response effect from quartile 4 to quartile 1, and also previous case-control studies failed to find a graded pattern with decreasing ADAMTS13 quartiles.14,15,25 This suggests that there is more a low-level threshold rather than a clear dose association for ADAMTS13 and ischemic stroke. For the association of continuous ADAMTS13 activity (per SD decrease) and stroke, we estimated whether the risks were influenced by outliers of ADAMTS13 activity by excluding the lowest and highest 1% and 2.5%. These data showed comparable risk estimates, as shown in Table 3, suggesting that there is not an important influence of outliers. High VWF:Ag levels have been shown to be associated with the risk of stroke in previous studies.5-7 To investigate whether stroke is also dependent on the interaction between VWF and ADAMTS13, we analyzed groups based on the combination of ADAMTS13 activity and VWF:Ag levels. Individuals with low ADAMTS13 activity and high VWF levels had the highest risk of ischemic stroke, suggesting that ADAMTS13 and VWF are independent risk factors. This finding is similar to that of 2 recent case-control studies in patients with cardiovascular disease.12,13 We observed no association between VWF:Ag levels and ADAMTS13 activity, which is consistent with previous reports.12,13,25,26 This might be explained by the fact that the ADAMTS13 activity levels measured in our study participants are apparently high enough for cleavage of plasma VWF. However, locally at the site of vessel damage, the reduced ADAMTS13 activity may lead to reduced cleavage of secreted large multimers of VWF and thereby contribute to local thrombus formation.13
A causal role of ADAMTS13 in the outcome of stroke has recently been suggested in patients with a congenital deficiency of ADAMTS13 (Upshaw-Schulman syndrome) and in animal studies. These patients without ADAMTS13 also experience ischemic stroke events.27,28 In addition, 2 independent groups reported larger infarctions in ADAMTS13-deficient mice in which ischemic stroke was induced experimentally.9-11 In addition, abolishment of the ADAMTS13 gene strongly accelerates atherosclerosis in a murine model,29,30 suggesting a role of ADAMTS13 in the progression of atherosclerosis. Infusion of recombinant human ADAMTS13 in wild-type mice with cerebral ischemia before reperfusion reduced the infarct size and improved the functional outcome.9 These studies suggest that recombinant human ADAMTS13 may have a role as a therapeutic agent in ischemic stroke patients31 ; however, this should be addressed in future clinical studies.
The measurement of ADAMTS13 in a large population-based cohort of nearly 6000 individuals allowed us to study the association between ADAMTS13 activity and several baseline characteristics and provides more insight into the pathophysiology of ADAMTS13. We observed a strong age dependency of ADAMTS13, indicating lower ADAMTS13 activity in the older and elderly population. In our population of individuals >55 years, the mean ADAMTS13 activity was 91.9%. ADAMTS13 activity was significantly lower in individuals of increasing age, which is consistent with other smaller studies.19,26,32,33 Although both age and ADAMTS13 activity are associated with the risk of stroke, we found that ADAMTS13 activity was associated with an increased risk of stroke also independent of age. Interestingly, other studies have revealed that even in children, low levels of ADAMTS13 are associated with stroke.15 In addition, we found an association between ADAMTS13 activity and sex. ADAMTS13 activity was higher in women than in men, as previously reported.19,33 We also observed that patients with diabetes had a higher activity of ADAMTS13. This is in contrast with a previous study that showed lower ADAMTS13 activity in patients with diabetes mellitus compared with controls.34 However, in that study a positive association between glycemic control, measured as HbA1c, and ADAMTS13 activity was reported. The authors suggested that this might be explained in these patients by the higher level of metabolic stress that increases the hepatic production and release of ADAMTS13 as a compensatory mechanism.34 We observed also a weak association between use of lipid-reducing agents and ADAMTS13. In addition, we found a positive association with total cholesterol, which confirms a previous study.26 Although ADAMTS13 activity was associated with multiple characteristics and drugs, the association between ADAMTS13 activity and ischemic stroke found in our study was independent of these variables.
The initial step in evaluation of a new marker is to examine whether the marker could predict development of future events. Although providing significant evidence of association is necessary, the key aspect is to show that a new risk marker improves predictions over the standard clinical risk assessment tools. When added to the traditional risk factors (age, sex, systolic blood pressure, treatment of hypertension, total and HDL cholesterol, smoking, and diabetes),23 ADAMTS13 improved the ischemic stroke risk predictions, as measured by an increase in model discrimination and moderate reclassification in the overall population and in persons categorized as having a risk between 5% and 7.5% by traditional cardiovascular risk factors, as depicted in Table 5. This is of importance because in these patients, preventive treatment is suggested to be considered.24 To allow for a more precise and clinically relevant interpretation of the contribution of ADAMTS13 activity to stroke risk prediction, we repeated the analysis for different traditional risk factors including systolic blood pressure, total and HDL cholesterol, smoking, and diabetes. We added each traditional risk factor to the model already containing other traditional risk factors and assessed improvements in model fit, discrimination, and reclassification for each traditional risk factor (data not shown). Our results confirmed that the contribution of ADAMTS13 to stroke risk prediction is comparable with those of systolic blood pressure, HDL cholesterol, and smoking.
The addition of VWF to the model did not improve the risk prediction. This might be explained by the fact that ADAMTS13 has a more important role in the prediction of stroke than VWF:Ag levels. We previously already published that the role of VWF:Ag in predicting stroke in this older and elderly population study was not very strong.5
The strength of our study is that this is a prospective study with nearly 6000 individuals and the role of ADAMTS13 in ischemic stroke, with a long follow-up of 10.7 years in a cohort that is representative of the Dutch population. A limitation of our study is that 23% of the incident strokes had to be classified as unspecified because of the lack of cerebral imaging. These strokes mainly occurred in the early phase of the study when patients were often not referred to the hospital. Because 80% to 90% of all strokes are ischemic,35,36 most of the unspecified strokes in our study will also be ischemic in origin. Another limitation is that ischemic stroke could only be subclassified in etiologic subgroups in a limited number of ischemic stroke patients. This limited our ability to study the importance of ADAMTS13 activity as a risk factor of various subtypes of ischemic stroke. We measured ADAMTS13 activity only at baseline because this is an observational study, and it would be of interest how ADAMTS13 levels change over time. Furthermore, a marker of stroke severity like the modified Rankin Score, was not determined in our study. Therefore we could not investigate whether ADAMTS13 activity is associated with stroke severity. However, a TIA is considered to be a less severe stroke and we found similar risk estimates of the association between ADAMTS13 activity and TIA or ischemic stroke. This suggests that there is no association between ADAMTS13 activity and stroke severity. Our study was performed in a population of predominantly Caucasians of ≥55 years who live in a middle-income district of Rotterdam, which limits the generalizability of our results. We did not take into account any competing morbidity and mortality in the analyses or clinical variables during follow-up. We could have possibly obtained more insight on the association between ADAMTS13 and stroke if multiple measurements over time would have been available.
In conclusion, low ADAMTS13 activity was significantly associated with the risk of ischemic stroke, independent of age, sex, and established cardiovascular risk factors over a median of 10.7 years of follow-up. Addition of ADAMTS13 activity improved the accuracy of risk predictions for ischemic stroke beyond the traditional risk factors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Schmidt, S. Kaufmann, G. Schrenk, and J. Schreiner from Baxter Innovations GmbH for their excellent technical assistance.
This study was supported in part by research funding from Baxter Innovations GmbH, Vienna, Austria (F.W.G.L.); the Dutch Heart Foundation (DHF-2007B159) (F.W.G.L.); an Erasmus MC Fellowship 2013 (M.A.I.); and the AXA Research Fund (M.K.).
Authorship
Contribution: M.A.H.S. designed the study, performed laboratory and statistical analysis, interpreted data, and wrote the manuscript; M.P.M.d.M. conceived of and designed the study, performed statistical analysis, interpreted data, and revised the manuscript; M.L.P.P. and M.K. performed statistical analysis, interpreted data, and revised the manuscript; A.H. and P.J.K. interpreted data and revised the manuscript; P.L.T., H.R., and F.S. designed the laboratory studies, supervised the laboratory analysis, discussed the data, and revised the manuscript; M.A.I. designed the study, performed statistical analysis, interpreted data, and revised the manuscript; F.W.G.L. conceived of and designed the study, interpreted data, and wrote and critically revised the manuscript; and all authors gave final approval of the manuscript; and M.A.H.S. and M.L.P.P. both had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: F.W.G.L. has served on advisory boards of CSL Behring and Baxter in the past. P.L.T., H.R., and F.S. are full-time employees of Baxter Innovations GmbH, Vienna, Austria. The remaining authors declare no competing financial interests.
Correspondence: Frank W. G. Leebeek, Erasmus University Medical Center, Department of Hematology, Room Na-820, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.