After stem cell transplants, steroid treatment severely damages T-cell responses to cytomegalovirus and abrogates the beneficial effect of adoptively transferred virus-specific T cells. In this issue of Blood,Menger et al describe a clinically applicable technique to inactivate the glucocorticoid receptor with transcription activator–like effector nuclease (TALEN) to render T cells resistant to steroid-induced apoptosis while retaining antiviral functions.1
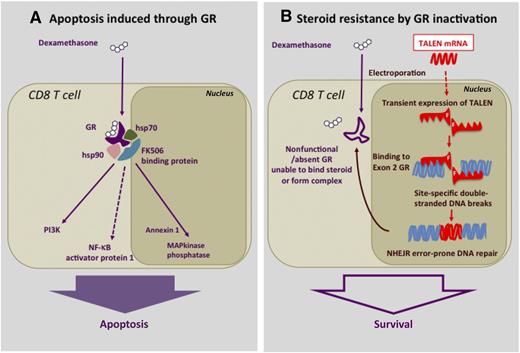
Dexamethasone-induced apoptosis in T cells and its disruption by TALEN. (A) Pathway of steroid-induced apoptosis in T cells through the GR. —–, indirect effects. (B) Steroid resistance induced by electroporation of GR-Ex2–specific TALEN mRNA to block GR synthesis through disruption of the GR exon 2. F, Fokl nuclease domain for DNA cleavage; NHEJR, nonhomologous end-joining recombination.
Dexamethasone-induced apoptosis in T cells and its disruption by TALEN. (A) Pathway of steroid-induced apoptosis in T cells through the GR. —–, indirect effects. (B) Steroid resistance induced by electroporation of GR-Ex2–specific TALEN mRNA to block GR synthesis through disruption of the GR exon 2. F, Fokl nuclease domain for DNA cleavage; NHEJR, nonhomologous end-joining recombination.
Corticosteroids such as methylprednisone are essential and frequently used drugs for the control of life-threatening complications of allogeneic hematopoietic stem cell transplantation (HSCT) such as graft-versus-host disease (GVHD). Steroids, in the high doses that are typically required, have a devastating immunosuppressive effect in such already immune-deficient individuals. All immune cells possess a cytosolic glucocorticoid receptor (GR) rendering them susceptible to the effects of steroids.2 In particular, T lymphocytes are highly sensitive to therapeutic doses of glucocorticoids, which on binding to the cytosolic complex of the GR, heat shock proteins 70 and 90 (hsp70 and hsp90), and the FK506 binding protein, activate transfer of the GR to the nucleus, leading to activation of apoptotic pathways through annexin 1 and mitogen-activated protein (MAP) kinase, as well as indirectly through phosphatidylinositol 3-kinase (PI3K) and nuclear factor κB (NF-κB)3,4 (see figure, panel A).
After HSCT, steroid treatment of GVHD further weakens immune responses already compromised by immune dysfunction from GVHD. A frequent consequence of GVHD and its treatment is thus the reactivation of the DNA viruses cytomegalovirus (CMV), Epstein-Barr virus, BK polyomavirus, and adenovirus. In particular, CMV reactivation frequently complicates steroid-dependent acute GVHD.5 This presents a therapeutic dilemma for the transplant physician faced with the incompatible needs of controlling the alloreaction with immunosuppression while at the same time trying to preserve immunity against an equally life-threatening viral infection. Although antiviral drugs now make it more feasible to control CMV reactivation under steroid treatment, they do not guarantee control of CMV in every situation. Indeed, CMV and other viral infections still contribute significantly to mortality after HSCT.6 It is now clear that CMV-competent CD8+ and CD4+ T lymphocytes are the critical components of the immune control of reactivating viruses such as CMV. For this reason, a number techniques have been developed to increase cell-mediated immunity against CMV by adoptive transfer of virus-specific T cells generated from the stem cell donor.7 Abundant data attest to the efficacy of such adoptively transferred CMV-specific T cells in controlling CMV reactivation and preventing lethal infection. However, although commonly used immunosuppressive agents, such as the calcineurin inhibitors and mycophenolate, probably do not interfere with CMV control by adoptively transferred T cells, steroids have a devastating impact, rapidly reducing the number of circulating virus-competent lymphocytes and promoting viral proliferation.8 Clearly, the ability to use steroids and at the same time deliver potent antiviral cell-mediated immunity would fulfill an important therapeutic need.
An international collaboration of colleagues from London and Birmingham, United Kingdom; Paris, France; and Seattle, Washington, have now achieved this goal. In the paper, Menger et al describe the successful use of TALEN gene transfer to inactivate the GR on CMV-specific CD8+ T cells to render them steroid resistant.1 The technique involves the selection and expansion from donor blood of CMV-specific CD8+ T cells recognizing the immunodominant HLA A2-restricted CMV-pp65 9-mer peptide. These highly specific oligoclonal T-cell populations are then electroporated with a TALEN mRNA selected to bind specifically to the GR gene by virtue of their highly specific 17-bp targeting domains. TALEN causes site-specific double-stranded DNA breaks in the GR gene and then triggers repair through nonhomologous end joining recombination. Such recombinations are error prone and result in the inactivation of the GR gene by random insertion or deletion, altering the reading frame and leading to the failure to form a functional GR protein (see figure, panel B). The authors first tested the system in the T2 cell line and showed that, after selection by culture in dexamethasone, the TALEN-modified cells could proliferate normally in medium containing high concentrations of dexamethasone. Repeat experiments with CMV-specific CD8 T-cell lines showed that TALEN-electroporated and dexamethasone-selected CMV-specific T cells retained full cytotoxicity against pp65-expressing targets when cultured in dexamethasone, whereas nonelectroporated controls in dexamethasone did not even survive adequately to test their function. Recognizing that the downside to their approach would be the risk of conferring steroid resistance on CD8 T cells that cause GVHD, the authors also studied the effect of GR-suppressed T cells in a humanized mouse xeno-GVHD model. CD8 T cells caused severe GVHD, which could be abrogated by steroids in this model. However, GVHD in mice receiving TALEN-electroporated T cells was completely unresponsive to steroid treatment.
What are the clinical implications from this technology? Although the approach appears highly intricate, the components of the process are already being developed in clinical practice. A number of clinical approaches to generating CMV-specific T cells (and indeed for several other viruses) are in clinical trials,7 and TALEN electroporation is easy to scale up. The pathway to clinical translation and early phase trials thus appears uncomplicated. In addition, the absence of viral vectors and the short survival of TALEN in the targeted cells are attractive to regulatory bodies concerned with potential risks of gene modification in transduced cells.9 A proof-of-principle study of TALEN-modified T cells in HSCT recipients would be a significant next step for broader applications of gene silencing with TALEN. Nevertheless, there are some important concerns about modification of T cells to resist the very agents that might be needed to suppress unwanted and off-target cytotoxicity, and the authors rightly point out that incorporation of a suicide gene into the final cell product would be required in circumstances where desired specificity of the T-cell population cannot be guaranteed.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal