Key Points
The G9a methyltransferase inhibitor UNC0638 increased pancellular expression of HbF to levels greater than 30% in adult human erythroblasts.
UNC0638 altered globin locus epigenetic status/protein occupancy favoring LCR interaction with fetal genes at the expense of adult genes.
Abstract
Induction of fetal hemoglobin (HbF) production in adult erythrocytes can reduce the severity of sickle cell disease and β-thalassemia. Transcription of β-globin genes is regulated by the distant locus control region (LCR), which is brought into direct gene contact by the LDB1/GATA-1/TAL1/LMO2-containing complex. Inhibition of G9a H3K9 methyltransferase by the chemical compound UNC0638 activates fetal and represses adult β-globin gene expression in adult human hematopoietic precursor cells, but the underlying mechanisms are unclear. Here we studied UNC0638 effects on β-globin gene expression using ex vivo differentiation of CD34+ erythroid progenitor cells from peripheral blood of healthy adult donors. UNC0638 inhibition of G9a caused dosed accumulation of HbF up to 30% of total hemoglobin in differentiated cells. Elevation of HbF was associated with significant activation of fetal γ-globin and repression of adult β-globin transcription. Changes in gene expression were associated with widespread loss of H3K9me2 in the locus and gain of LDB1 complex occupancy at the γ-globin promoters as well as de novo formation of LCR/γ-globin contacts. Our findings demonstrate that G9a establishes epigenetic conditions preventing activation of γ-globin genes during differentiation of adult erythroid progenitor cells. In this view, manipulation of G9a represents a promising epigenetic approach for treatment of β-hemoglobinopathies.
Introduction
In humans, the β-globin cluster contains fetal Aγ- and Gγ-globin and adult δ- and β-globin genes. Around the time of birth, fetal hemoglobin (HbF) is almost completely replaced by adult hemoglobin (HbA) containing 2 β-globin chains. Based upon this developmental transition in hemoglobin production, mutations in the β-globin gene locus can cause a variety of hemoglobinopathies including sickle cell disease and β-thalassemia. One longstanding goal for developing treatments for these β-hemoglobinopathies is the reactivation and increased expression of HbF in adult erythroid cells.1 Therefore, considerable research effort has been focused upon understanding the mechanisms that underlie γ-globin gene repression during the developmental switch between HbF and HbA that could suggest new therapeutic approaches for these diseases.
Expression of β-globin genes is regulated by physical interactions between gene promoters and the locus control region (LCR) enhancer.2,3 Experiments using RNA interference have shown that this interaction is facilitated by the LDB1/LMO2/GATA-1/TAL1 erythroid-specific protein complex (LDB1 complex).4-6 The LDB1 complex occupies the LCR and the β-globin gene promoter and provides chromatin loop formation between them through interaction between LDB1 homodimerization domains.7,8
Mouse β-globin genes are also regulated by the G9a/EHMT2 H3K9 histone methyltransferase.9,10 G9a contains a SET domain responsible for histone H3K9 mono- and di-methylation associated with repression of gene expression.11 Interestingly, recent observations support the view that G9a can play a role in activation of gene expression independently from its repressive methyltransferase activity.12 In human cells, G9a functions as a stable heteromeric complex with a related protein, GLP (EHMT1).13,14 UNC0638 specifically inhibits methyltransferase activity of G9a and GLP, causing a strong decrease in bulk H3K9me2 and reactivation of G9a-silenced genes in mouse embryonic stem cells.15 UNC0638 treatment of CD34+ hematopoietic progenitor cells delayed adoption of differentiated phenotypes, suggesting an important role for G9a in lineage specification.16 Moreover, brief treatment of these cells with UNC0638 activated fetal γ-globin genes in parallel with repression of adult δ- and β-globin genes, reversing the normal sequence of events that occurs late in erythroid differentiation. The mechanistic role of G9a in epigenetic regulation of the β-globin locus remains unclear. Here, we investigated the role of G9a in silencing fetal γ-globin genes and activation of adult δ- and β-globin genes during ex vivo differentiation of CD34+ adult hematopoietic progenitor cells. We found that UNC0638 treatment acts primarily upon erythroblasts as they acquire a glycophorin A positive (GPA+) phenotype in response to erythropoietin, and we show that G9a is directly involved in epigenetic repression of the human γ-globin genes.
Methods
Cell culture
All related studies were performed after human subject review and National Institutes of Health Institutional Review Board approval. These studies were conducted in accordance with the Declaration of Helsinki. CD34+ cells were cultured ex vivo in a 3-phase, serum-free culture system for 21 days as described previously.17 UNC0638 (Sigma Aldrich, St. Louis, MO) was dissolved in dimethylsulfoxide and added at designated concentrations.16
Flow cytometry analyses
Cell differentiation of the erythroid populations in Figure 1 were monitored with antibodies against CD71 (MHCD7104) and glycophorin A (MHGLA01) obtained from Invitrogen (Grand Island, NY) on culture days 14 and 21 using the BD FACSAria I flow cytometer (BD Biosciences, San Jose, CA), as previously described.18 Cells that had a fluorescence of more than 2 standard deviations above the unstained control cells were defined as positive. For fetal hemoglobin analysis, 1 million cells were washed in 0.1% bovine serum albumin/phosphate-buffered saline and fixed in 3.7% paraformaldehyde (Electron Microscopy Science, Hatfield, PA), washed again in 0.1% bovine serum albumin/phosphate-buffered saline followed by permeabilization in 1% Triton X-100 (Invitrogen) before immunostaining with an antibody directed against HbF (MHFH04; Invitrogen).
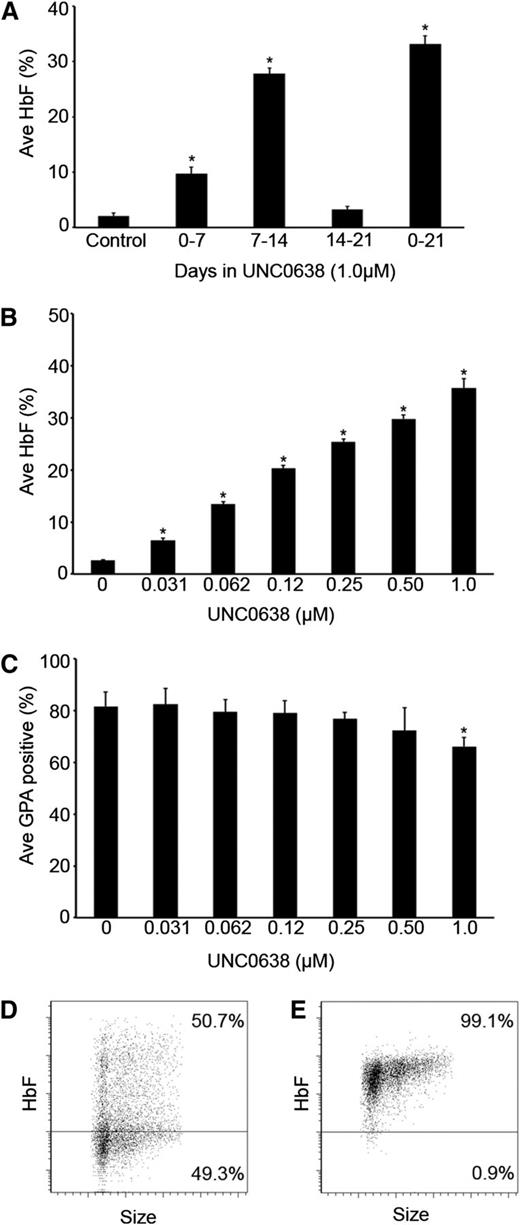
Inhibition of G9a methyltransferase activity by UNC0638 in adult human erythrocytes stimulates fetal hemoglobin production. (A) The percentage of HbF, relative to total hemoglobin (HbF + HbA), measured in culture day 21 control, phase 1, phase 2, phase 3, and phase 1-3 cells treated with 1.0 µM UNC0638. (B) The percentage of HbF, relative to total hemoglobin (HbF + HbA), in culture day 21 control and phase 2 cells treated with depicted concentrations of UNC0638. (C) Quantitative analysis of the percentage of GPA+ cells measured by fluorescence-activated cell sorter analysis of control and phase 2 cells treated with depicted concentrations of UNC0638 at culture day 14. Representative flow cytometric dot plots of (D) control cells and (E) 1.0 µM UNC0638 phase 2 treated cells at culture day 21 stained for fetal hemoglobin. In panels A-C, error bars indicate standard error of the mean (SEM); n = 3 independent donors for each condition. *Student t test values of P < .05 compared with control. Ave, average.
Inhibition of G9a methyltransferase activity by UNC0638 in adult human erythrocytes stimulates fetal hemoglobin production. (A) The percentage of HbF, relative to total hemoglobin (HbF + HbA), measured in culture day 21 control, phase 1, phase 2, phase 3, and phase 1-3 cells treated with 1.0 µM UNC0638. (B) The percentage of HbF, relative to total hemoglobin (HbF + HbA), in culture day 21 control and phase 2 cells treated with depicted concentrations of UNC0638. (C) Quantitative analysis of the percentage of GPA+ cells measured by fluorescence-activated cell sorter analysis of control and phase 2 cells treated with depicted concentrations of UNC0638 at culture day 14. Representative flow cytometric dot plots of (D) control cells and (E) 1.0 µM UNC0638 phase 2 treated cells at culture day 21 stained for fetal hemoglobin. In panels A-C, error bars indicate standard error of the mean (SEM); n = 3 independent donors for each condition. *Student t test values of P < .05 compared with control. Ave, average.
High-performance liquid chromatography for HbA and HbF
Protein samples were prepared and hemoglobin profiles were analyzed as described previously.17
RT-qPCR
RNA samples were prepared and analyzed by RT-qPCR as described previously.17 Primers, probes and conditions used to perform reverse transcription quantitative polymerase chain reaction (RT-qPCR) assays for α-globin, β-globin, and γ-globin gene transcripts were previously described.19 Analysis of gene expression was carried out using the following Assays-on-Demand Gene Expression Products (Applied Biosystems, Grand Island, NY): TAL1 (Hs01097987_m1); LMO2 (Hs00153473_m1); LDB1 (Hs01597578_m1); KLF1 (Hs00610592_m1); GATA-1 (Hs01085822_g1); EHMT2 (Hs00198710_m1); EHMT1 (Hs00964325_m1); and BCL11A (Hs00256254_m1). Comparison of standard curves from a plasmid DNA encoding each globin template was used to calculate individual globin copy numbers.
ChIP
Chromatin immunoprecipitation (ChIP) was performed as described20 using published primers.21 Primers used for γ-globin genes do not distinguish between Aγ- and Gγ-globin genes and the values presented in Figures 2-4 are the average signal from both. The data for H3K9me2, H3K27me2, and H3K36me3 were normalized to the H3 signal. Antibodies used included H3K9me2 (ab1220), H3K36me3 (ab9050), H3 (ab1791), and G9a (ab40542) (Abcam, Cambridge, MA); H3K27me2 (07-452; Millipore, Temecula, CA); LDB1 (sc-11198), TAL1 (sc-12984), and GATA-1 (sc-265) (Santa Cruz Biotechnology, Dallas, TX); LMO2 (AF2726) (R&D Systems, Minneapolis, MN); control goat (sc-2028), mouse (sc-2025), and rabbit (sc-2027) immunoglobulin G (Santa Cruz Biotechnology).
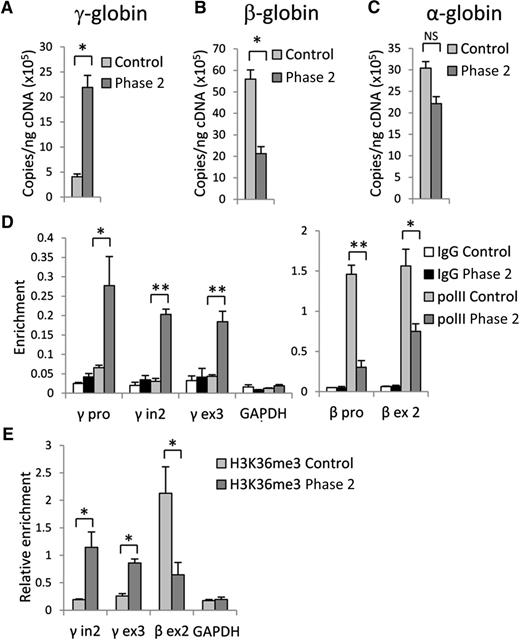
Inhibition of G9a results in upregulation of γ-globin and downregulation of β-globin genes expression. RT-qPCR analysis of (A) γ-globin, (B) β-globin, and (C) α-globin gene expression in control and phase 2–treated cells at culture day 14. (D) RNA PolII and (E) H3K36me3 occupancy at γ- and β-globin genes in control and phase 2–treated cells at culture day 14. Error bars indicate SEM; n = 3 independent donors for each condition. Student t test values of *P < .05 or **P < .01 compared with control. cDNA, complementary DNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IgG, immunoglobulin G.
Inhibition of G9a results in upregulation of γ-globin and downregulation of β-globin genes expression. RT-qPCR analysis of (A) γ-globin, (B) β-globin, and (C) α-globin gene expression in control and phase 2–treated cells at culture day 14. (D) RNA PolII and (E) H3K36me3 occupancy at γ- and β-globin genes in control and phase 2–treated cells at culture day 14. Error bars indicate SEM; n = 3 independent donors for each condition. Student t test values of *P < .05 or **P < .01 compared with control. cDNA, complementary DNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IgG, immunoglobulin G.
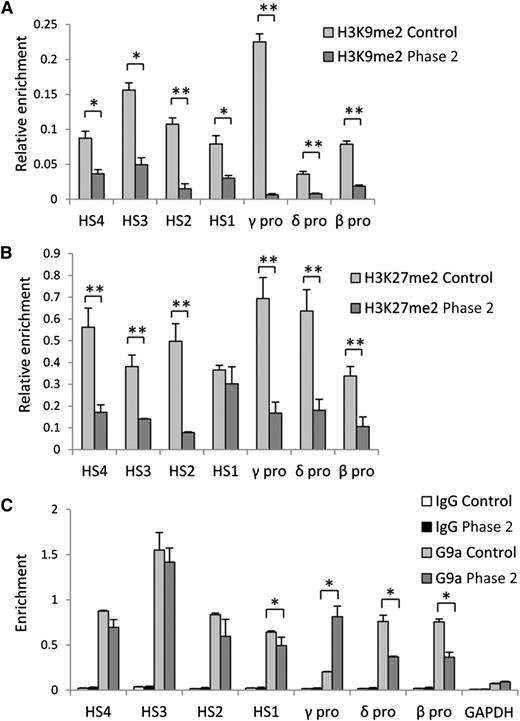
G9a methyltransferase activity is required for H3K9me2 and G9a distribution in the β-globin locus. (A) H3K9me2, (B) H3K27me2, and (C) G9a occupancy determined by ChIP at LCR HS sites and γ-, δ-, and β-globin gene promoters in control and phase 2–treated cells at culture day 14. Error bars indicate SEM; n = 3 independent donors for each condition. Values are compared with the value for control. *P < .05, **P < .01 by Student t test.
G9a methyltransferase activity is required for H3K9me2 and G9a distribution in the β-globin locus. (A) H3K9me2, (B) H3K27me2, and (C) G9a occupancy determined by ChIP at LCR HS sites and γ-, δ-, and β-globin gene promoters in control and phase 2–treated cells at culture day 14. Error bars indicate SEM; n = 3 independent donors for each condition. Values are compared with the value for control. *P < .05, **P < .01 by Student t test.
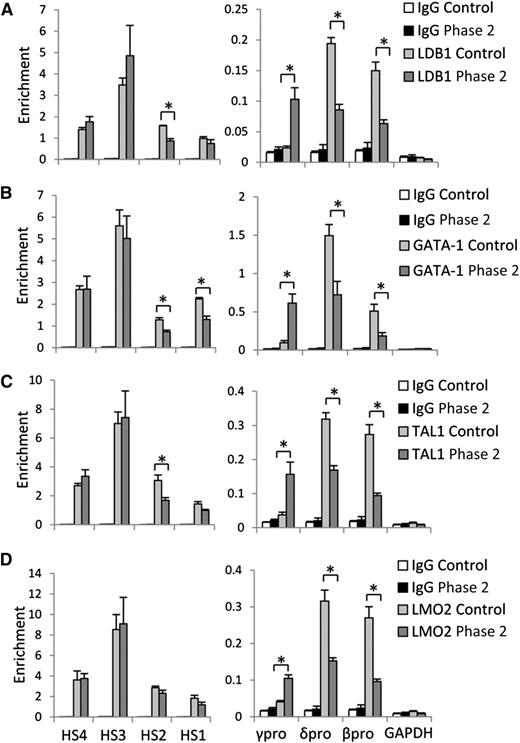
H3K9me2 protects γ-globin gene promoter from LDB1/GATA-1/TAL1/LMO2 protein complex occupancy. (A) LDB1, (B) GATA-1, (C) TAL1, and (D) LMO2 protein occupancy at LCR HS sites, and γ-, δ-, and β-globin gene promoters in control and phase 2–treated cells at culture day 14. Error bars indicate SEM; n = 3 independent donors for each condition. Values are compared with the value for control. *P < .05 by Student t test.
H3K9me2 protects γ-globin gene promoter from LDB1/GATA-1/TAL1/LMO2 protein complex occupancy. (A) LDB1, (B) GATA-1, (C) TAL1, and (D) LMO2 protein occupancy at LCR HS sites, and γ-, δ-, and β-globin gene promoters in control and phase 2–treated cells at culture day 14. Error bars indicate SEM; n = 3 independent donors for each condition. Values are compared with the value for control. *P < .05 by Student t test.
3C
Chromosome conformation capture (3C) assays were performed as described using EcoRI (New England Biolabs, Ipswich, MA) cleavage.8 Relative crosslinking between the anchor fragment and fragments of interest were analyzed by SYBR Green real-time qPCR using published primers.21 The primer used for detecting interaction with γ-globin genes does not distinguish between Aγ- and Gγ-globin genes, and the value presented in Figure 5 is the average signal from both. Interaction between 2 fragments within the α-tubulin gene was used as the internal normalization control for 3C. To eliminate variability between donor samples, interaction frequencies were normalized by interaction between the anchor fragment and the fragment encompassing the ε-gene.
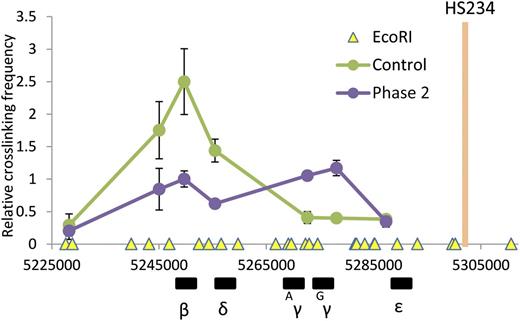
H3K9me2 protects γ-globin gene from looping with LCR. 3C assay measuring locus-wide crosslinking frequencies in control and phase 2–treated cells at culture day 14. The EcoRI fragments containing HSs of the LCR (orange bar) was used as the anchor region. The X-axis shows genomic coordinates and globin gene locations. EcoRI restriction sites are depicted by yellow triangles. Error bars indicate SEM; n = 3 independent donors for each condition.
H3K9me2 protects γ-globin gene from looping with LCR. 3C assay measuring locus-wide crosslinking frequencies in control and phase 2–treated cells at culture day 14. The EcoRI fragments containing HSs of the LCR (orange bar) was used as the anchor region. The X-axis shows genomic coordinates and globin gene locations. EcoRI restriction sites are depicted by yellow triangles. Error bars indicate SEM; n = 3 independent donors for each condition.
Results
G9a represses fetal hemoglobin production during adult human erythropoiesis
A 3-phase culture system was used for these studies, as previously described.17 During the first phase of culture (days 0-7), the CD34+ cells differentiate into progenitor cell populations that include CD36+ erythroblasts. For phase 2 of culture (days 7-14), erythropoietin is added to the culture medium causing proliferation and expression of GPA on the plasma membrane. During the final phase of culture (phase 3: days 14-21), the cells undergo further differentiation with terminal maturation resulting in about 20% enucleated cells. For initial screening of UNC0638, the compound at a concentration of 1.0 µM was added with fresh culture medium to a single phase (culture days 0, 7, or 14), or added to the fresh medium in all 3 culture phases.
The addition of UNC0638 caused the most pronounced increase in the percent of HbF during phase 2, and the effect was no greater if the drug was present throughout the culture period (Figure 1A). There was a modest rise in HbF with UNC0638 present during phase 1 only compared with control; however, when added during the third phase, there was no significant change in HbF when compared with the control. Based on the screening experiments, a dosed titration was performed during phase 2 with concentrations ranging from 4.0 to 0.031 µM UNC0638. Doses of 2.0 and 4.0 µM were cytotoxic to the cells, preventing further studies (data not shown). As the UNC0638 concentration was titrated from 0.031 to 1.0 µM, a corresponding gain in the percent of HbF was observed (Figure 1B; see supplemental Figure 1A on the Blood Web site). UNC0638 effects also included some inhibition of differentiation when compared with controls as evidenced by a reduced proportion of GPA+ cells on culture day 14 (Figure 1C; supplemental Figure 1B). At culture day 21, the cells were stained for fetal hemoglobin to determine its distribution. When compared with the control, UNC0638 resulted in pancellular expression of HbF (Figure 1D-E).
G9a methyltransferase activity is required for γ-globin gene repression
Because high-level, pancellular HbF expression was detected in cells after the addition of 1.0 µM UNC0638 during phase 2, further investigation was mainly focused upon this culture condition. Using RT-qPCR, we confirmed that control cells express mostly the β-globin gene with low-level γ-globin gene expression (Figure 2A-B). UNC0638 treatment led to a 5-fold increase in γ-globin gene expression, along with an accompanying decrease in β-globin gene expression. A significant change in α-globin gene expression was not detected (Figure 2C). RNA levels of several transcription factors involved in the regulation of globin genes were also measured and demonstrated that inhibition of G9a had variable effects upon their expression (supplemental Figure 2). These results support a major role of G9a methyltransferase activity in regulation of β-globin gene expression.
The activation of γ-globin and repression of β-globin genes by UNC0638 should be reflected by changes in RNA polymerase II (PolII) occupancy and the presence of the elongation-associated H3K36me3 histone mark within the active gene.22 ChIP using control cells with antibodies against PolII or H3K36me3 revealed PolII occupancy at the β-globin gene promoter and H3K36me3 within the gene body without significant accumulation at the γ-globin genes. UNC0638 treatment caused a strong increase of PolII and H3K36me3 signals at the γ-globin genes with a concomitant decrease at the β-globin gene (Figure 2D-E). This observation is consistent with reduced recruitment of PolII and H3K36me3 to the adult mouse β-globin genes after G9a reduction using small hairpin RNAs in MEL cells.9 Together, these results document that accumulation of HbF in adult erythroid cells in which G9a methyltransferase activity has been inhibited by UNC0638 results from significant repression of the β-globin gene and activation of γ-globin gene expression.
Inhibition of G9a methyltransferase activity causes eviction of H3K9me2 and redistribution of G9a protein in the β-globin locus
Because inhibition of G9a methyltransferase activity by UNC0638 caused a widespread decrease in euchromatin-associated H3K9me2 in hematopoietic stem cell progenitors,16 we predicted that changes in H3K9 methylation could be responsible for the globin gene expression switch upon UNC0638 treatment during differentiation. To test this hypothesis, we performed ChIP assays with an antibody against H3K9me2. In control cells, the strongest H3K9me2 signal was detected at the silenced γ-globin gene promoters consistent with the repressive role of H3K9me223 (Figure 3A). After UNC0638 treatment in phase 2, the H3K9me2 signal was significantly reduced across the β-globin locus. Moreover, the strongest (35-fold) decrease in H3K9me2 was observed at the activated γ-globin gene promoters, consistent with the absence of this mark at active human fetal globin genes.24 Additionally, H3K27me2 distribution was investigated because it has been reported to serve a repressive role with respect to εy-globin expression in mouse MEL cells, which is relieved by G9a reduction.9 H3K27me2 was reduced across the locus in UNC0638-treated cells but to a more modest extent than for H3K9me2 and can still be detected at the γ-globin promoter (Figure 3B). UNC0638 treatment during phase 1 also reduced H3K9me2 occupancy when measured on culture day 7 (supplemental Figure 3). However, after 7 additional culture days in the absence of UNC0638, reductions in H3K9me2 occupancy relative to controls were no longer detected.
Given that G9a can also act as an activator of gene expression independently from its methyltransferase activity,9,12 we investigated how UNC0638 treatment affects G9a distribution across the β-globin locus using ChIP. In control cells, strong G9a signals were detected at the LCR hypersensitive (HS) sites and promoters of actively transcribed adult globin genes (Figure 3C). UNC0638 treatment did not affect G9a distribution at the LCR HS sites, but caused a decrease of G9a occupancy at the δ- and β-globin gene promoters and an increase at activated γ-globin gene promoters. However, G9a transcription was not affected (supplemental Figure 2). Taken together, these data suggest that inhibition of G9a methyltransferase activity results in elimination of the repressive H3K9me2 chromatin mark from the γ-globin gene promoters and a shift of G9a-associated activator activity from adult to fetal globin genes.
Occupancy by the LDB1 complex at activated fetal γ-globin gene promoters
Chromatin looping mediated by the LDB1 complex underlies activation of human globin gene expression.6,21 Because inactivation of G9a enhances chromatin opening and binding of transcription factors,25,26 we next examined how UNC0638 affects LDB1 complex distribution in the β-globin locus using ChIP with antibodies to the component members of the complex. Figure 4 shows that GATA-1, TAL1, LMO2, and LDB1 are strongly detected at the LCR HS sites in control and UNC0638-treated cells. The LDB1 complex occupied actively expressed δ- and β-globin gene promoters with an undetectable signal at the silent γ-globin gene promoter in control cells. Inactivation of G9a methyltransferase activity led to strong accumulation of LDB1 complex members at activated γ-globin gene promoters with a reciprocal decrease at the promoters of adult globin genes. These results demonstrate that G9a methyltransferase activity prohibits LDB1 complex binding to γ-globin gene promoters and facilitates occupancy at adult globin gene promoters in adult erythroid cells.
G9a inactivation induces γ-globin/LCR looping
Given the role of the LDB1 complex in facilitating long-range interactions in the β-globin locus8,21,27 and its redistribution after G9a inactivation, looping between an anchor LCR fragment and the β-globin genes was examined using the 3C assay. UNC0638 treatment established an interaction between the LCR and the γ-globin genes that is absent in control cells (Figure 5). Additionally, interactions between the LCR and adult β-globin genes were significantly decreased after treatment, consistent with establishment of an overall locus looping configuration favoring LCR interaction with the fetal genes at the expense of the adult globin genes. In summary, inactivation of G9a methyltransferase activity facilitates de novo formation of long-range contact between γ-globin genes and the LCR with activation of γ-globin gene expression.
Discussion
Here we show that inactivation of G9a methyltransferase activity in differentiating adult erythroid cells by UNC0638 causes a dose-dependent accumulation of HbF. Unexpectedly, the response to treatment with UNC0638 was highly dependent upon the differentiation stage of the cells. The strongest accumulation of HbF was detected when UNC0638 was applied during phase 2 (days 7-14), suggesting that the G9a-dependent decision regarding whether fetal or adult hemoglobin would be produced is made at the period when erythroid differentiation is induced by addition of erythropoietin. Cells treated with UNC0638 during phase 3 did not increase HbF, suggesting that the pattern of globin gene transcription is not reversible during this stage or that the robustness of globin gene transcription is diminishing or both. There was a small increase in HbF observed at culture day 21 for cells incubated with UNC0638 during the first 7 days of culture (phase 1) when CD34+ cells are differentiating into CD36+ erythroblasts, but before the onset of high-level globin gene transcription. This result may be explained by the observation that although UNC0638 effectively reduced H3K9me2 across the β-globin locus during phase 1, the modification was strongly reestablished at the γ-globin promoters and at HS3 after removal of the drug (supplemental Figure 3).
G9a establishment of H3K9me2 is localized in silenced domains of euchromatin.23 The strongest accumulation of H3K9me2 in the β-globin locus of adult erythroid cells was detected at the silenced γ-globin genes in agreement with previous observations.24 Higher H3K9me2 at the fetal compared with the adult genes was also reported in primary human bone marrow erythroblasts in the absence of ex vivo culture.28 Inhibition of G9a methyltransferase activity caused a strong decrease of H3K9 dimethylation throughout the locus, but this was especially notable at the γ-globin gene promoter associated with reactivation of γ-globin gene expression, further supporting a repressive role for G9a in regulation of hemoglobin production. Surprisingly, the decrease in H3K9 dimethylation at the adult globin gene promoters after UNC0638 treatment was associated with repression of these genes suggesting that H3K9me2 does not play a significant role in regulation of the adult β-globin genes. This notion is supported by previous experiments demonstrating that activation of the mouse adult β-globin gene is not associated with changes in H3K9me2 levels at the gene promoter.9 Besides H3K9me2, we observed a moderate decrease in H3K27me2 across the β-globin locus after inhibition of G9a methyltransferase activity. Whether this is a direct or indirect effect remains unclear as recent data suggest G9a methyltransferase activity is not directly linked to the H3K27me2 mark in vivo.29-31
Besides the well-established role of G9a in gene repression, several lines of evidence support its methyltransferase-independent role in activation of gene expression.12 In the mouse β-globin locus, G9a activates adult globin gene expression independently from methyltransferase activity through interaction with mediator complex.9,10 Consistent with these observations, G9a occupied promoters of actively transcribed adult globin genes where H3K9me2 was low in control cells. Unexpectedly, UNC0638 treatment caused elimination of G9a from the adult gene promoters but drastically increased its occupancy at γ-globin gene promoters. Given that G9a is involved in preinitiation complex formation at globin gene promoters, the observation of G9a redistribution was further supported by redistribution of PolII occupancy from repressed β-globin gene to activated γ-globin gene promoters. Thus, both the methyltransferase activity of G9a to establish gene silencing via H3K9me2 deposition and its methyltransferase-independent activity to recruit mediator and PolII are central to fetal-to-adult globin switching.
How G9a recruitment is altered as a result of UNC0638 treatment remains an open question. G9a is recruited to cytosine guanine dinucleotide (CpG) islands genome-wide and spreads from these sites to establish H3K9me2 domains encompassing silenced chromatin.16 However, G9a is recruited to the mouse β-globin locus LCR HS2 site, which is not a CpG island, and spreads in the locus from that site.9 G9a occupancy at HS2 depends on NF-E2. Our data suggest that recruitment of G9a within the LCR and spreading through the human β-globin locus are unaffected by UNC0638 and loss of its methyltransferase activity. Rather, it is the specific localization of G9a at either fetal or adult globin genes that is affected by UNC0638, which correlates with gene expression. G9a also interacts with PolII9 and participates in a gene-activating complex with a mediator.10 We speculate that increased occupancy of G9a at the fetal γ-globin promoters is emblematic of occupancy by such activating complexes. However, the order of events in localization of this complex at the γ-globin promoters with respect to decreased H3K9me2, increased LDB1 complex occupancy, and LCR looping remains unclear. Further, because G9a also methylates nonhistone chromatin remodeling factors,32,33 regulation of other globin gene regulatory proteins might be considered.
Expression of both the adult and fetal globin genes is regulated by the LDB1-containing protein complex through facilitating loop formation between the LCR and expressed genes.5,6 There is a tight correlation between globin gene expression status, LDB1 protein complex occupancy, and looping with the LCR.21 Recently, the primary role of LDB1 in regulation of the globin gene expression was confirmed by artificial tethering of the LDB1 dimerization domain to silenced γ-globin gene promoters with subsequent activation of its expression in adult erythroid cells.27 In agreement with these observations, we found that H3K9me2 elimination from the γ-globin gene promoters facilitates LDB1 complex occupancy and loop formation between γ-globin gene promoters and the LCR at the expense of the adult δ- and β-globin promoters (Figure 6). A similar mechanism may be involved in regulation of the estrogen receptor–dependent bcl-2 gene.34 Inactivation of LSD1, responsible for elimination of H3K9 dimethylation from the bcl-2 gene promoter, inhibited estrogen receptor binding and loop formation between the bcl-2 gene and enhancer. These results illustrate that if γ-globin gene silencing is prevented, the LCR will favor interaction with them and activate their transcription, which is consistent with both competitive and autonomous models of fetal γ- and adult δ/β-globin gene transcription activation.35
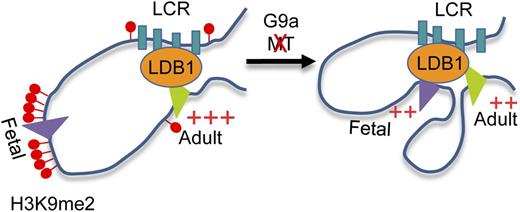
Model describing the role of G9a established H3K9me2 in regulation of fetal and adult β-globin genes expression in adult erythrocytes. G9a establishes H3K9me2-dependent repressive chromatin protecting the γ-globin gene promoters from interaction with the LDB1 complex and the LCR in adult erythroid cells. Inactivation of G9a methyltransferase activity (MT) causes elimination of H3K9me2 from the γ-globin gene promoters, facilitating LDB1 complex occupancy and interaction with LCR.
Model describing the role of G9a established H3K9me2 in regulation of fetal and adult β-globin genes expression in adult erythrocytes. G9a establishes H3K9me2-dependent repressive chromatin protecting the γ-globin gene promoters from interaction with the LDB1 complex and the LCR in adult erythroid cells. Inactivation of G9a methyltransferase activity (MT) causes elimination of H3K9me2 from the γ-globin gene promoters, facilitating LDB1 complex occupancy and interaction with LCR.
Inhibition of G9a methyltransferase activity in adult erythroid cells of patients with hemoglobinopathies such as sickle cell disease and β-thalassemia should cause increased expression of HbF production, with subsequent beneficial therapeutic effect.36 Although poor in vivo pharmacokinetic properties of UNC0638 may preclude its use as a drug, additional G9a targeting compounds are being developed, including UNC0642, with similar inhibitory properties and improved in vivo stability.37 In addition to the identification and development of UNC0638-related compounds, recognition of the role of H3K9 methylation in globin gene regulation could advance mechanistic studies of existing or alternate drugs that augment HbF expression, including cytidine derivatives. Although 5-Aza-2′-deoxycytidine is thought to act primarily via DNA demethylation, its epigenetic effects include strong reduction of H3K9me2 histone marks at gene promoters.38 In baboons treated with 5-Aza-2′-deoxycytidine by subcutaneous injection, changes in several histone modifications at the globin genes were detected, but H3K9me2 was not studied.39 Alternatively, loss of G9a/GLP activity may direct DNA methylation40 or affect the activity of LSD1, another therapeutic target for induction of fetal hemoglobin.41 Of note, RE1 silencing transcription factor–mediated gene repression involves colocalization of G9a, LSD1, and histone deacetylases with other chromatin remodeling proteins.42 Our data suggest that histone-modifying effects of G9a, as well as its functional interplay with other proteins present at the globin locus, should be explored in the context of HbF expression.43-45 An understanding of how the methyltransferase-dependent and methyltransferase-independent functions of G9a are linked at the level of chromatin to influence globin locus epigenetics can further advance efforts toward the development of HbF-inducing drugs.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Authorship
Contribution: I.K., C.B., J.F.d.V., Y.T.L., and M.K. performed experiments and analyzed data; A.D. and J.L.M. conceived, assisted, and directed research; and I.K., C.B., A.D., and J.L.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffery L. Miller, Molecular Medicine Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 10 Center Dr, Building 10, Room 9N311, Bethesda, MD 20892; e-mail: jm7f@nih.gov; and Ann Dean, Laboratory of Cellular and Developmental Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 50 South Dr, Building 50, Room 3154, Bethesda, MD 20892; e-mail: anndean@helix.nih.gov.
References
Author notes
I.K. and C.B. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal