In this issue of Blood, Sugata et al report that vaccination against human T-cell leukemia virus type 1 (HTLV-1) basic leucine zipper (bZIP) factor (HBZ) could be used for immunotherapy in adult T-cell leukemia-lymphoma (ATL) patients.1
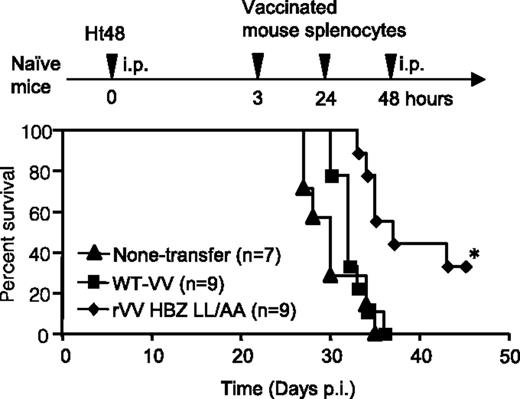
HBZ-specific effector cells improved the survival ratio in an HBZ-induced ATL model. Naïve mice were inoculated intraperitoneally with lymphoma cells expressing HTLV-1 HBZ and then with peptide-stimulated splenocytes from rVV-HBZ–vaccinated mice. Survival curves were analyzed by the Kaplan-Meier method, and statistical differences were calculated by the log-rank test; *P < .05 by the log-rank test. See Figure 4B in the article by Sugata et al that begins on page 1095.
HBZ-specific effector cells improved the survival ratio in an HBZ-induced ATL model. Naïve mice were inoculated intraperitoneally with lymphoma cells expressing HTLV-1 HBZ and then with peptide-stimulated splenocytes from rVV-HBZ–vaccinated mice. Survival curves were analyzed by the Kaplan-Meier method, and statistical differences were calculated by the log-rank test; *P < .05 by the log-rank test. See Figure 4B in the article by Sugata et al that begins on page 1095.
The HTLV-1 human oncogenic retrovirus was discovered more than 30 years ago. It infects 5 to 10 million individuals and is the etiologic agent of both neurologic (eg, HTLV-1–associated myelopathy/tropical spastic paraparesis [HAM/TSP]) and hematologic (eg, ATL) diseases. Although a recent report suggests that it possible to decrease HTLV-1 proviral load in carriers at risk for developing a disease by using a combination of reverse transcriptase and histone deacetylase inhibitors,2 most treatments, when delivered to ATL and HAM/TSP patients, show a modest rate of success.3,4 The risk of developing ATL is linked to infection early in life. However, transmission could be prevented by screening blood products and avoiding maternal transmission via breast milk. It may be possible to create an anti-HTLV-1 therapeutic vaccine for those who are infected and who develop ATL. Given the extreme genetic stability of HTLV-1, which is linked to the clonal expansion of infected cells rather than to the use of the viral reverse transcriptase,5 it was originally believed that developing an anti-HTLV-1 vaccine would be an easy task.6 The viral envelope is required to bind to the cell receptor(s) and elicits both a humoral and a cellular immune response in infected individuals. In addition, neutralizing antibodies directed toward the viral envelope have been described. Thus, viral envelope-recombinant vaccinia or adenoviruses as well as viral envelope naked DNA and chimeric peptides were generated and used to immunize mice, rats, rabbits, or monkeys in the 1990s and early 2000s.7,8 However, partial protection was observed in only a limited number of animals after challenge with HTLV-1–infected cells. An animal model in which ATL or HAM/TSP development could be observed at a reasonable frequency was also lacking at that time and did not allow the development of therapeutic vaccine.
Adding another level of complexity, it is well established that HTLV-1 viral expression is extremely low in human ATL patients because of provirus deletion or methylation of the 5′ long terminal repeat. This issue needs to be addressed when designing a therapeutic vaccine. The discovery of HBZ, which is encoded by an antisense viral transcript,9 challenged this paradigm. Indeed, HBZ is expressed in infected cells isolated from asymptomatic carriers but, more importantly, in malignant cells from ATL patients. In addition, HBZ transgenic mice develop lymphoma. Thus, this protein is oncogenic and might be considered a target for anti-ATL immunotherapy. Interestingly enough, recent in silico results also suggest that the immune response against HBZ influences proviral load.10
The study by Sugata et al1 aimed to determine whether a recombinant vaccinia virus expressing HBZ as an antigen (rVV-HBZ) would elicit specific T-cell responses and whether this vaccine could be used to treat mice inoculated with HBZ lymphoma cells. The HBZ sequence was modified so that the protein could not activate the transforming growth factor β/Smad pathway. First, Sugata et al showed that anti-HBZ cytotoxic T lymphocytes can be obtained in vivo in mice (as well as in rhesus macaques), although HBZ immunogenicity is weak and requires several boosts. Importantly, anti-HBZ CD8+ produces interferon gamma and tumor necrosis factor α. These CD8+ cells recognize and kill mouse T cells pulsed with HBZ peptides and mouse T cells transduced with an HBZ expression vector. More interestingly, CD4+ T cells isolated from HBZ-transgenic animals were efficiently eliminated when transferred to HBZ-vaccinated mice. Thus, rVV-HBZ induces a cytotoxic response against cells that express HBZ in vivo.
More importantly, Sugata et al1 showed that adoptive transfer of splenocytes obtained from rVV-HBZ–vaccinated mice significantly improved the survival of animals inoculated with transformed T cells that express only HBZ, as do most ATL cells (see figure). This indicates that rVV-HBZ elicits a T-cell response that is sufficient to eliminate HBZ lymphoma cells in vivo.
Finally, Sugata et al identified an HBZ peptide (amino acids 157-176) that could be used to generate a peptide-based vaccine. Altogether, the results from Sugata et al are important with respect to the generation of an anti-ATL therapeutic vaccine. Now the efficiency of the vaccine needs to be tested against primary human ATL cells.
Conflict-of-interest disclosure: The author declares no competing financial interests.