Key Points
BAX 855, a pegylated full-length rFVIII with extended half-life, was highly effective in the prevention and treatment of bleeding events.
No subjects receiving BAX 855 developed FVIII inhibitory antibodies nor experienced unexpected adverse events.
Abstract
Current management of hemophilia A includes prophylaxis with factor VIII (FVIII) replacement every 2 to 3 days. BAX 855, Baxalta’s pegylated full-length recombinant FVIII (rFVIII), was designed to increase half-life and, thus, reduce the frequency of prophylactic infusions while maintaining hemostatic efficacy. BAX 855 was evaluated in previously treated patients with severe hemophilia A who were aged 12 to 65 years. A phase 1 study compared the pharmacokinetic (PK) profile of BAX 855 with that of licensed rFVIII (Advate). In a pivotal study, the annualized bleeding rate (ABR), PK parameters, and efficacy of bleeding treatment were assessed. In the phase 1 study, the mean half-life (T1/2) and the mean residence time of BAX 855 compared with Advate were 1.4- to 1.5-fold higher. These results were confirmed in the pivotal study. The pivotal study met its primary endpoint: Prophylaxis with BAX 855 resulted in an ABR that was significantly lower than half the ABR of on-demand treatment (P < .0001). The median ABR was 1.9, and 39.6% of compliant subjects had no bleeding episodes during prophylaxis, whereas subjects treated on-demand had a median ABR of 41.5. BAX 855 was also efficacious for the treatment of bleeding episodes, with 95.9% of bleeding episodes treated with 1 to 2 infusions and 96.1% having efficacy ratings of excellent/good. No FVIII inhibitory antibodies or safety signals were identified. These studies provide evidence that BAX 855 was safe and efficacious for on-demand treatment and prophylaxis administered twice weekly in patients with hemophilia A. The trials were registered at www.clinicaltrials.gov as #NCT01736475 and #NCT01599819.
Introduction
Hemophilia A is an X chromosome-linked recessive, congenital bleeding disorder characterized by a deficiency of functional coagulation factor VIII (FVIII), resulting in a prolonged clotting time that leads to frequent bleeding in joints and soft tissue. Patients with hemophilia A who adopt a prophylactic regimen can reduce their bleeding rate and, thus, reduce the probability of developing chronic arthropathy, which leads to disability. Current management of severe hemophilia A (FVIII < 1% of normal) includes on-demand treatment of bleeding episodes and prophylaxis.1-3 The average half-life (T1/2) of FVIII products is in the range of 10 to 14 hours4-8 ; thus, current prophylactic regimens require infusion of FVIII every other day, or every 2 to 3 days when based on each patient’s individual pharmacokinetic (PK) profile.9 An extended half-life rFVIII product would offer additional therapeutic options for patients, permitting a reduction in the frequency of prophylactic infusions. Several strategies for creating longer-acting replacement factors are in development, including modifications to the FVIII molecule, such as pegylation, glycopegylation, recombinant fusion to immunoglobulin (Ig) Fc, modification of the amino acid sequence to create sites for site-directed pegylation, and disulfide linkage between its light and heavy chains.10-12 Chemical modification with pegylation is a well-established method of improving the PK profile by extending T1/2 and circulation of therapeutic proteins.13 With the goal of reducing the number of infusions required per week for prophylaxis, a pegylated recombinant FVIII-rurioctacog alfa pegol (BAX 855) was built to extend FVIII half-life on the manufacturing platform of Advate, a full-length, unmodified rFVIII (Baxalta US Inc., Westlake Village, CA). BAX 855 is manufactured by covalently binding a branched polyethylene glycol (PEG) reagent with a molecular weight of 20 kDa to Advate, using proprietary technology from Nektar Therapeutics (San Francisco, CA). BAX 855 is created through controlled pegylation, in which approximately 60% of the PEG chains are localized to the B-domain.14
Two clinical studies of BAX 855 were conducted in male patients with severe hemophilia A. A phase 1 study assessed the safety and PK profile of BAX 855 compared with Advate at 2 doses (30 ± 3 and 60 ± 6 IU/kg; all doses are IU/kg bodyweight). This was followed by a pivotal study undertaken to evaluate the efficacy, PK profile (45 ± 5 IU/kg), and safety of BAX 855 administered as twice-weekly prophylactic or as on-demand therapy in severe hemophilia A. This report describes the efficacy and safety results of these 2 studies.
Methods
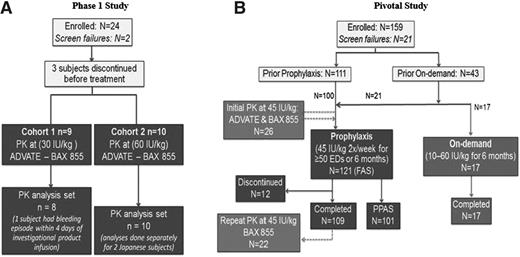
The studies received ethical approval by institutional review boards according to International Conference on Harmonisation Good Clinical Practice guidelines, which are based on principles that have their origin in the Declaration of Helsinki.15 Written informed consent was obtained from all subjects at the time of enrollment. Independent Data Monitoring Committees monitored the subjects’ safety throughout the studies. Study flow and subject disposition are presented in Figure 1.
Both clinical studies were open label with no randomization. In the phase 1 study (A), the sequence of treatment was Advate first, followed by BAX 855 for each subject in each cohort. Nine subjects in the 30 IU/kg group were treated first. After review of the data from the first cohort and approval by the Data Monitoring Committee, treatment of 10 subjects in the 60 IU/kg group commenced. Only 1 treated subject was excluded from the PK analysis set as a result of a bleeding episode within 4 days of infusion. In the pivotal study (B), treatment assignment depended on the subjects’ prior treatment: Subjects previously receiving prophylaxis were assigned to the prophylaxis group. The first 17 subjects who previously received on-demand treatment were assigned to the on-demand group, then additional subjects were assigned to the prophylaxis arm. The PK subset comprised 26 subjects in the prophylaxis group. Twelve subjects discontinued during prophylaxis: 1 for a surgical procedure, 1 because of screen failure, 2 because of discontinuation by the subject, 4 because of an adverse event, and 4 for protocol violation.
Both clinical studies were open label with no randomization. In the phase 1 study (A), the sequence of treatment was Advate first, followed by BAX 855 for each subject in each cohort. Nine subjects in the 30 IU/kg group were treated first. After review of the data from the first cohort and approval by the Data Monitoring Committee, treatment of 10 subjects in the 60 IU/kg group commenced. Only 1 treated subject was excluded from the PK analysis set as a result of a bleeding episode within 4 days of infusion. In the pivotal study (B), treatment assignment depended on the subjects’ prior treatment: Subjects previously receiving prophylaxis were assigned to the prophylaxis group. The first 17 subjects who previously received on-demand treatment were assigned to the on-demand group, then additional subjects were assigned to the prophylaxis arm. The PK subset comprised 26 subjects in the prophylaxis group. Twelve subjects discontinued during prophylaxis: 1 for a surgical procedure, 1 because of screen failure, 2 because of discontinuation by the subject, 4 because of an adverse event, and 4 for protocol violation.
Study designs
A phase 1, prospective, open label, cross-over, dose-escalation study evaluated safety and PK profile of single doses of BAX 855 compared with single doses of Advate at 2 doses. In the 30 ± 3 IU/kg group, subjects were infused with Advate with collection of 7 postinfusion blood samples for FVIII measurement during a 48-hour period. After a 72-hour washout period, the same dose of BAX 855 was administered with the collection of 14 postinfusion blood samples for FVIII measurement during a 168-hour period (refer to supplemental Data, available on the Blood Web site). After review of the data and approval by the Data Monitoring Committee, the 60 ± 6 IU/kg group underwent the same evaluations.
A pivotal phase 2/3, multicenter, open-label study evaluated the efficacy and safety of prophylactic and on-demand treatment, as well as the PK profile of BAX 855. Subjects were assigned to treatments on the basis of their prestudy FVIII treatment regimen; however, once 17 subjects were assigned to on-demand treatment, subsequent subjects were assigned to the prophylactic group, regardless of their previous treatment regimen. The prophylactic treatment regimen of a 45 ± 5 IU/kg twice-weekly dose was designed to ensure that a majority of subjects maintained FVIII levels above 1% at all times. This dose was selected on the basis of calculations that took into account the 1.4- to 1.5-fold extended half-life of BAX 855 compared with Advate observed in the phase 1 study. The prophylactic dose was administered for 50 or more exposure days (EDs) or 6 months ± 2 weeks. On-demand therapy with BAX 855 at a dose of 10 to 60 ± 5 IU/kg occurred for 6 months ± 2 weeks. A subgroup of 25 subjects who were to receive prophylactic treatment was included in a PK evaluation. Advate was used as a PK comparator: subjects first received 45 ± 5 IU/kg Advate with a collection of 10 postinfusion blood samples for FVIII measurement during a 56-hour period, followed by a minimum washout period of 72 hours and then BAX 855 at 45 ± 5 IU/kg with the collection of 12 postinfusion blood samples during a 96-hour period. A repeat PK followed after subjects completed at least 50 EDs after a minimum washout period of 84 hours with the collection of 12 postinfusion blood samples during a 96-hour period (refer to supplemental Data). At baseline, the number of target joints (a joint with 3 or more spontaneous bleeding episodes in any consecutive 6-month period) was recorded. Arthropathy was considered present if it was reported in the subject’s medical history or if the patient had undergone joint surgery.
Patient population
The key criteria for inclusion were similar in both studies: a diagnosis of severe hemophilia A (untreated FVIII activity < 1%), age 12 to 65 years, and previous treatment with plasma-derived FVIII or rFVIII concentrates for 150 or more documented EDs. Subjects must also have had no history of FVIII inhibitory antibodies (0.4 BU or higher, using the Nijmegen modification of the Bethesda assay, or 0.6 BU or higher, using the Bethesda assay) at any time before screening and no detectable FVIII inhibitory antibodies, as confirmed by the central laboratory at screening. A complete list of inclusion and exclusion criteria is provided in the supplemental Data.
Outcome measures
The primary outcome measure of the phase 1 study was the number and proportion of subjects experiencing serious and nonserious adverse events (SAEs/AEs). PK parameters as assessed by the 1-stage clotting assay and the chromogenic assay were secondary outcome measures in both studies. The pivotal study’s primary efficacy outcome measure was to compare annualized bleeding rates (ABRs) between the prophylactic and on-demand treatment groups. Secondary efficacy outcome measures in the pivotal study included evaluating BAX 855 for treatment of bleeding episodes as “excellent or good,” according to a 4-point rating scale (described in the supplemental Data), the number of infusions to treat bleeding episodes, the interval between bleeding episodes, the weight-adjusted consumption of BAX 855, and patient-reported outcomes (PROs), including the Short Form 36 (SF-36) validated questionnaire to assess quality of life (QoL) and the Haemo-SYM questionnaire, a hemophilia-specific validated tool.16
Safety outcome measures in both studies were tolerability and immunogenicity, including the development of inhibitory antibodies to FVIII and binding antibodies (IgG and IgM) to FVIII, PEG-FVIII, and PEG, analyzed using validated enzyme-linked immunosorbent assays. Antibodies to Chinese hamster ovary proteins (considered potential impurities from the manufacturing process) were assessed only in the pivotal study. Safety outcome measures for both studies included the occurrence of AEs and SAEs, as well as changes in vital signs and clinical laboratory parameters.
Statistical analysis
In the phase 1 study, the number and proportion of subjects experiencing SAEs and nonserious AEs from the first treatment up to 4 weeks ± 4 days after infusion of BAX 855 were summarized. All nonserious AEs that occurred during or after treatment were presented descriptively. In a post hoc analysis, Spearman's rank correlation test was performed to evaluate the correlation between the T1/2 and the clearance of FVIII and von Willebrand factor (VWF).
In both studies, PK parameters were calculated by the noncompartmental analysis module of Phoenix WinNonlin V6.2.1 (Pharsight Corporation, Cary, NC).
In the pivotal study, approximately 132 subjects were planned for treatment, with the aim of achieving approximately 104 subjects evaluable from the prophylactic group and 15 subjects from the on-demand group. For the primary outcome measure, prophylaxis was considered successful if the upper limit of the 95% confidence interval (CI) for the ratio between treatment means did not exceed 0.5 (corresponding to a 50% reduction of the mean ABR compared with on-demand treatment). A negative binomial model accounting for prophylaxis vs on-demand, presence or absence of target joints at screening, and age at screening as fixed effects and the duration of the observation period as an offset was used. The primary outcome analysis was performed on the full analysis set, which included all subjects assigned to treatment, and the per protocol analysis set (PPAS) (Table 1). Success of bleeding episode treatment was defined as a rating of excellent or good. The 95% confidence interval of mean success rates was calculated within a generalized estimating equation model, pooling both treatment regimens, and the lower limit of the confidence interval was compared with a 70% threshold (the lowest clinically acceptable success rate). Power calculations for the analyses of the primary outcome and key secondary outcome measures were performed by Monte Carlo simulations (details are provided in the supplementary Data).
Criteria for inclusion in the PPAS of the pivotal study
| Regimen . | Treatment . | Criterion . | n (%) . |
|---|---|---|---|
| Prophylaxis (N = 120) | Prophylaxis | Infusion interval of 5 or more days did not occur more than 3 times | 107 (89.2) |
| The daily dose was below 35 IU/kg in no more than 10% of the infusions | 116 (96.7) | ||
| The daily dose was above 55 IU/kg in no more than 10% of the infusions | 118 (98.3) | ||
| No dose adjustments resulting from bleeding episodes | 118 (98.3) | ||
| All of above | 101 (85.8) | ||
| Breakthrough bleeding episode | Dose to treat the bleed was below 5 IU/kg for minor bleed, below 10 IU/kg for moderate bleed, or below 25 IU/kg for a major bleed for no more than 5 bleeds (minor/moderate/major taken together) | 120 (100.0) | |
| On-demand (N = 17) | Bleeding episode | Dose to treat the bleed was below 5 IU/kg for minor bleed, below 10 IU/kg for moderate bleed, or below 25 IU/kg for a major bleed for no more than 5 bleeds (minor/moderate/major taken together) | 17 (100.0) |
| Regimen . | Treatment . | Criterion . | n (%) . |
|---|---|---|---|
| Prophylaxis (N = 120) | Prophylaxis | Infusion interval of 5 or more days did not occur more than 3 times | 107 (89.2) |
| The daily dose was below 35 IU/kg in no more than 10% of the infusions | 116 (96.7) | ||
| The daily dose was above 55 IU/kg in no more than 10% of the infusions | 118 (98.3) | ||
| No dose adjustments resulting from bleeding episodes | 118 (98.3) | ||
| All of above | 101 (85.8) | ||
| Breakthrough bleeding episode | Dose to treat the bleed was below 5 IU/kg for minor bleed, below 10 IU/kg for moderate bleed, or below 25 IU/kg for a major bleed for no more than 5 bleeds (minor/moderate/major taken together) | 120 (100.0) | |
| On-demand (N = 17) | Bleeding episode | Dose to treat the bleed was below 5 IU/kg for minor bleed, below 10 IU/kg for moderate bleed, or below 25 IU/kg for a major bleed for no more than 5 bleeds (minor/moderate/major taken together) | 17 (100.0) |
One hundred twenty-one subjects were assigned to the prophylaxis regimen (full analysis set); however, there were only 120 subjects (safety analysis set) who received prophylactic treatment with BAX 855, of whom 101 qualified for the PPAS. One subject received Advate for PK and then discontinued the study. For guidelines for treatment of bleeds and definition of minor, moderate, or major bleed, refer to the supplemental Data.
N, subjects who received treatment; n, subjects who met criterion for inclusion in the PPAS.
Descriptive statistical analyses were presented for bleeding episodes, PK parameters, and safety. PK parameters were also analyzed for correlations with ABR and VWF:Ag concentrations, using Spearman’s rank correlation coefficients. Statistical analysis of the proportion of subjects developing inhibitory antibodies to FVIII was conducted using the Clopper Pearson technique. Changes from baseline to end of treatment in the QoL and PRO assessments were estimated using the Hodges-Lehmann estimator and were compared between prophylaxis and on-demand treatment in a hierarchical testing scheme.
Results
Patients
Demographics are presented in Table 2. In both studies, distributions of characteristics were comparable for each treatment group. In the phase 1 study, 19 subjects received single infusions of BAX 855 and Advate for PK analysis at 5 investigative sites. In the pivotal study, 138 patients at 72 investigative sites were assigned to treatment groups and 137 subjects received at least 1 dose of BAX 855 (1 was treated only with Advate), including 120 subjects who received prophylactic treatment (99 of whom had received prior prophylactic FVIII treatment and 21 who were previously treated on-demand) and 17 subjects who received on-demand treatment (previously treated on-demand). Twenty-seven subjects from the prophylactic group were evaluated for PK. The median age for both studies was 29 years. All subjects were male, and most were white (75.2%) or Asian (24.1%); 1 was black/African American. One hundred twenty-six (126) subjects completed the pivotal study.
Subject demographics in subjects who received BAX 855
| Parameter . | Phase 1 study* (N = 19) . | Pivotal study prophylaxis (N = 120) . | Pivotal study on-demand (N = 17) . | Pivotal study all (N = 137) . |
|---|---|---|---|---|
| Median age (minimum-maximum), y | 29 (18-60) | 28 (12-58) | 32 (13-56) | 29 (12-58) |
| Median weight (minimum-maximum), kg | 82.6 (52.5-128.0) | 73.0 (39.5-137.5) | 77.0 (48.0-91.0) | 73.0 (39.5-137.5) |
| Race, n (%) | ||||
| White | 16 (84.2) | 92 (76.7) | 11 (64.7) | 103 (75.2) |
| Asian | 2 (10.5) | 27 (22.5) | 6 (35.3) | 33 (24.1) |
| Black/African American | 0 (0.0) | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Presence of target joints, n (%) | NA | 78 (65.0) | 15 (88.2) | 93 (67.9) |
| Presence of hemophilic arthropathy, n (%) | NA | 72 (60.0) | 8 (47.1) | 80 (58.4) |
| Hepatitis C virus antibody positive, n (%) | NA | 64 (53.3) | 12 (70.6) | 76 (55.5) |
| Parameter . | Phase 1 study* (N = 19) . | Pivotal study prophylaxis (N = 120) . | Pivotal study on-demand (N = 17) . | Pivotal study all (N = 137) . |
|---|---|---|---|---|
| Median age (minimum-maximum), y | 29 (18-60) | 28 (12-58) | 32 (13-56) | 29 (12-58) |
| Median weight (minimum-maximum), kg | 82.6 (52.5-128.0) | 73.0 (39.5-137.5) | 77.0 (48.0-91.0) | 73.0 (39.5-137.5) |
| Race, n (%) | ||||
| White | 16 (84.2) | 92 (76.7) | 11 (64.7) | 103 (75.2) |
| Asian | 2 (10.5) | 27 (22.5) | 6 (35.3) | 33 (24.1) |
| Black/African American | 0 (0.0) | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Presence of target joints, n (%) | NA | 78 (65.0) | 15 (88.2) | 93 (67.9) |
| Presence of hemophilic arthropathy, n (%) | NA | 72 (60.0) | 8 (47.1) | 80 (58.4) |
| Hepatitis C virus antibody positive, n (%) | NA | 64 (53.3) | 12 (70.6) | 76 (55.5) |
Two subjects participated in both studies.
N, number of subjects; NA, not applicable (data not collected).
Data generated from 2 Japanese subjects in the phase 1 study are not included in the report. An analysis of the 1-stage clotting assay results for these subjects revealed a number of outliers and therefore were not considered in the overall interpretation of PK data for BAX 855 and Advate.
Efficacy of prophylactic treatment
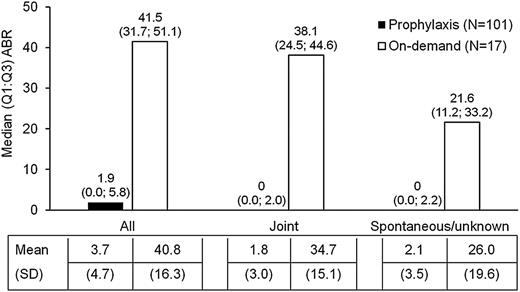
The efficacy of prophylaxis was assessed in the pivotal study only. The study met its primary endpoint for prophylactic treatment compared with on-demand treatment, using a negative binomial model analysis, which demonstrated a more than 50% reduction in ABR for the prophylactic group (P < .0001). A 90% reduction in ABR was observed in the prophylactic group compared with the on-demand group. Descriptive statistics also showed a much lower median (quartile 1; quartile 3 [Q1; Q3]) ABR for the prophylactic group (1.9 [0.0; 5.8]) compared with the on-demand treatment group (41.5 [31.7; 51.1]). A lower ABR was observed in the prophylactic treatment group regardless of bleeding type or etiology: joint ABRs were 0.0 (0.0; 2.0) during prophylaxis compared with 38.1 (24.5; 44.6) for on-demand treatment, and spontaneous/unknown ABRs were 0.0 (0.0; 2.2) during prophylaxis compared with 21.6 (11.2; 33.2) for the on-demand treatment (Figure 2).
In a descriptive analysis of 118 subjects in the PPAS, the median (Q1; Q3) and mean (SD) ABRs were computed for the prophylactic group vs the on-demand group, for all, joint and spontaneous bleeding episodes.
In a descriptive analysis of 118 subjects in the PPAS, the median (Q1; Q3) and mean (SD) ABRs were computed for the prophylactic group vs the on-demand group, for all, joint and spontaneous bleeding episodes.
Subjects received a median (Q1; Q3) of 44.6 (42.6; 46.8) IU/kg of BAX 855 per prophylactic infusion. The mean (SD) reduction in dosing frequency from prestudy prophylaxis was 26.7% (27.9), with a reduction of 33.7% (2.5; 36.7). Furthermore, 70.4% of subjects treated prophylactically were able to reduce the frequency of dosing from their prestudy prophylactic treatment regimens by 30% or more, which is approximately equivalent to at least 1 fewer prophylactic infusion per week when using BAX 855 for prophylaxis. There was a median of 1.96 prophylactic infusions per week, indicating that BAX 855 prophylaxis was administered twice weekly. There was a negative correlation between ABR and T1/2 (−0.49 at the initial PK and −0.42 at the repeat PK), suggesting that subjects with a shorter circulation time had a higher ABR.
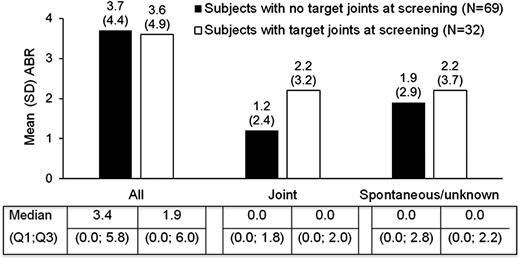
The mean (±SD) joint ABR in subjects in the prophylactic group presenting with target joints at the time of screening was higher (2.2 ± 3.2) than in those without target joints (1.2 ± 2.4) (Figure 3). As there were only 2 subjects in the on-demand group without target joints, a comparison of ABR between those with and without target joints is limited. During prophylaxis, 1 subject developed a new target joint and 1 subject had a target joint revert to a nontarget joint, whereas 5 subjects treated on-demand developed new target joints.
Mean annualized bleeding rates (ABRs) in subjects who received prophylactic treatment with BAX 855 are depicted by target joint status at screening. There were 101 subjects in the PPAS, including 69 subjects with no target joints at screening and 32 subjects with target joints at screening. Median ABR values are also presented.
Mean annualized bleeding rates (ABRs) in subjects who received prophylactic treatment with BAX 855 are depicted by target joint status at screening. There were 101 subjects in the PPAS, including 69 subjects with no target joints at screening and 32 subjects with target joints at screening. Median ABR values are also presented.
In the prophylactic group, there were approximately twice as many subjects with arthropathy (67 subjects) at screening compared with those without (34 subjects), and there was a higher mean (SD) joint ABR in the subjects with arthropathy (2.1 [3.2]) than in subjects without (1.4 [2.6]).
Approximately 60% of patients receiving prophylaxis achieved 5 months or longer between any hemorrhages, including 39.6% with zero bleeding events. For joint bleeds, approximately 80% of patients achieved 6 months or longer between episodes, including 57.4% with zero joint bleeding events. The observed frequency of bleeding was higher at longer than 96 hours after prophylactic infusions than observed at earlier times after infusions; however, this represents a period that is out of compliance with the prophylactic dosing regimen (Table 3).
Summary of bleeding episodes by time after prophylactic BAX 855 infusions
| Cause of bleeding episode parameter . | Time since last prophylactic infusion . | ||||
|---|---|---|---|---|---|
| ≤24 h . | >24 to ≤48 h . | >48 to ≤72h . | >72 to ≤96 h . | >96 h . | |
| NA | |||||
| Number of infusions in analysis | 5907 | 5862 | 5763 | 4297 | 1338 |
| Spontaneous/ unknown | |||||
| Number of bleeding episodes | 26 | 40 | 42 | 25 | 24 |
| Bleeding episodes per prophylactic infusion | 0.0044 | 0.0068 | 0.0073 | 0.0058 | 0.0179 |
| Injury | |||||
| Number of bleeding episodes | 21 | 30 | 30 | 9 | 15 |
| Bleeding episodes per prophylactic infusion | 0.0036 | 0.0051 | 0.0052 | 0.0021 | 0.0112 |
| All | |||||
| Number of bleeding episodes | 47 | 70 | 72 | 34 | 39 |
| Bleeding episodes per prophylactic infusion | 0.0080 | 0.0119 | 0.0125 | 0.0079 | 0.0291 |
| Cause of bleeding episode parameter . | Time since last prophylactic infusion . | ||||
|---|---|---|---|---|---|
| ≤24 h . | >24 to ≤48 h . | >48 to ≤72h . | >72 to ≤96 h . | >96 h . | |
| NA | |||||
| Number of infusions in analysis | 5907 | 5862 | 5763 | 4297 | 1338 |
| Spontaneous/ unknown | |||||
| Number of bleeding episodes | 26 | 40 | 42 | 25 | 24 |
| Bleeding episodes per prophylactic infusion | 0.0044 | 0.0068 | 0.0073 | 0.0058 | 0.0179 |
| Injury | |||||
| Number of bleeding episodes | 21 | 30 | 30 | 9 | 15 |
| Bleeding episodes per prophylactic infusion | 0.0036 | 0.0051 | 0.0052 | 0.0021 | 0.0112 |
| All | |||||
| Number of bleeding episodes | 47 | 70 | 72 | 34 | 39 |
| Bleeding episodes per prophylactic infusion | 0.0080 | 0.0119 | 0.0125 | 0.0079 | 0.0291 |
A bleeding episode at more than 96 hours could occur only if the subject deviated from the protocol-specified infusion interval, as 96 hours was the maximum. The analysis was performed on the full analysis set, which included subjects with prophylactic infusion intervals for 5 days or longer.
Efficacy of bleeding treatment
The efficacy of treatment was assessed in the pivotal study in all patients treated for bleeding (Table 4). Subjects were treated with a median (Q1; Q3) dose of BAX 855 of 30.87 (21.2; 45.2) IU/kg per episode and 29.19 (22.3; 44.0) IU/kg for the maintenance of hemostasis, which were additional infusions administered within 48 hours after a treatment. The study met its secondary efficacy endpoint. The rate of success of bleeding treatment (rated excellent or good) was 97%, with a corresponding 95% confidence interval ranging from 94% to 98%, which was thus significantly larger than 70% (P < .0001). Of 518 bleeding episodes reported during the study, treatment was rated excellent or good for 91.6%, and 95.9% were treated with 1 or 2 infusions. The dose per infusion used to treat bleeding episodes increased with bleeding severity (Table 4). Compared with the results for all bleeding episodes, the efficacy of treatment was similar for joint and nonjoint bleeding episodes (supplemental Data).
Efficacy of treatment of bleeding episodes in the pivotal study
| . | Treatment of bleeding episodes (N = 518*) . |
|---|---|
| Number of infusions to treat bleeding episodes, n (%) | |
| 1 infusion | 443 (85.5) |
| 2 infusions | 54 (10.4) |
| Total (1 or 2 infusions) | 497 (95.9) |
| Median (Q1 ; Q3) dose per infusion to treat a bleeding episode, IU/kg | |
| All bleeds/severities (N = 518) | 29.0 (20.0; 39.2) IU/kg |
| Minor (N = 245) | 25.5 (16.9;37.6) IU/kg |
| Moderate (N = 238) | 30.9 (23.0; 43.1) IU/kg |
| Severe/major (N = 35) | 36.4 (29.0; 44.5) IU/kg |
| Hemostatic efficacy rating, n (%) | |
| Excellent or good | 498 (96.1) |
| Infusions to treat a bleeding episode mean | |
| Number of infusions (SD) | 1.2 (0.7) |
| . | Treatment of bleeding episodes (N = 518*) . |
|---|---|
| Number of infusions to treat bleeding episodes, n (%) | |
| 1 infusion | 443 (85.5) |
| 2 infusions | 54 (10.4) |
| Total (1 or 2 infusions) | 497 (95.9) |
| Median (Q1 ; Q3) dose per infusion to treat a bleeding episode, IU/kg | |
| All bleeds/severities (N = 518) | 29.0 (20.0; 39.2) IU/kg |
| Minor (N = 245) | 25.5 (16.9;37.6) IU/kg |
| Moderate (N = 238) | 30.9 (23.0; 43.1) IU/kg |
| Severe/major (N = 35) | 36.4 (29.0; 44.5) IU/kg |
| Hemostatic efficacy rating, n (%) | |
| Excellent or good | 498 (96.1) |
| Infusions to treat a bleeding episode mean | |
| Number of infusions (SD) | 1.2 (0.7) |
PPAS for patients in prophylaxis and on-demand groups (N = 118). For PPAS criteria, refer to Table 1.
PKs
In the phase 1 study, subjects received a single dose of BAX 855 for the PK assessment. The range of total dose per PK infusion was: 29.4 to 31.3 IU/kg in the 30 IU/kg group and 54.0 to 60.0 IU/kg in the 60 IU/kg group. In the pivotal study, the range of BAX 855 PK was 40.2 to 54.8 IU/kg for the initial assessment and 38.9 to 48.5 IU/kg for the repeat.
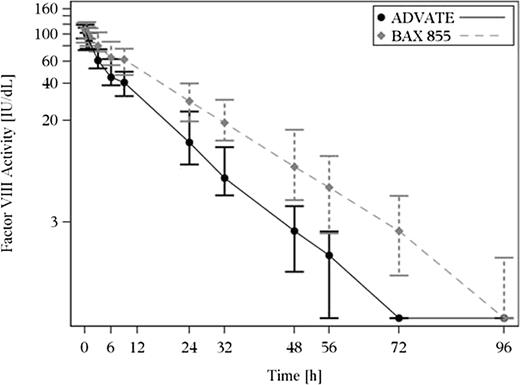
In both studies, mean T1/2 and mean residence time (MRT) were longer, mean clearance (CL) was lower, mean area under the curve (AUC0-∞) was greater, and mean incremental recovery (IR) was similar for BAX 855 compared with Advate at the same dose level. At the initial PK assessment for BAX 855 in the pivotal study, the mean T1/2 increased by 1.4-fold (Table 5). PK curves demonstrate an extended PK profile for BAX 855 compared with Advate in Figure 4. The MRT increased by 1.5-fold, and AUC0-∞ by 1.9-fold, compared with Advate, using the 1-stage clotting assay. As expected, the ratios between a repeat PK (after 50 or more EDs) and the initial PK profile confirmed that individual PK parameters for BAX 855 remained consistent over time, indicating that repeated exposure did not affect the PK profile of BAX 855. In the phase 1 study, mean fold increases in MRT with BAX 855 compared with Advate were 1.4-fold in the 30 IU/kg group and 1.5-fold in the 60 IU/kg group, and the mean fold increases in T1/2 were 1.4-fold in the 30 IU/kg group and 1.5-fold in the 60 IU/kg group. PK results from the chromogenic assay were similar (data not shown).
BAX 855 vs Advate PKs in the pivotal study
| . | Before prophylactic treatment, mean (SD) . | After 6 mo of prophylaxis, mean (SD) . | |||
|---|---|---|---|---|---|
| Advate (N = 26) . | BAX 855 initial (N = 26) . | Ratio: BAX 855 initial/Advate . | BAX 855 final (N = 22) . | Ratio BAX 855 final/initial . | |
| T1/2 , h | 10.4 (2.2) | 14.3 (3.8) | 1.4 (0.25) | 16.0 (4.9) | 1.2 (0.47) |
| MRT, h | 12.9 (3.0) | 19.6 (5.3) | 1.5 (0.18) | 20.7 (4.8) | 1.1 (0.26) |
| AUC0-∞, IU⋅h/dL | 1168 (425) | 2073 (778) | 1.9 (0.91) | 2009 (631.53) | 1.1 (0.5) |
| CL, dL/(kg⋅h) | 0.0455 (0.0217) | 0.0276 (0.0203) | 0.613 (0.28) | 0.0247 (0.00823) | 1.0 (0.27) |
| IR, (IU/dL)/(IU/kg) | 2.37 (0.536) | 2.49 0.694) | 1.1 (0.36) | 2.3 (0.64) | 1.0 (0.22) |
| . | Before prophylactic treatment, mean (SD) . | After 6 mo of prophylaxis, mean (SD) . | |||
|---|---|---|---|---|---|
| Advate (N = 26) . | BAX 855 initial (N = 26) . | Ratio: BAX 855 initial/Advate . | BAX 855 final (N = 22) . | Ratio BAX 855 final/initial . | |
| T1/2 , h | 10.4 (2.2) | 14.3 (3.8) | 1.4 (0.25) | 16.0 (4.9) | 1.2 (0.47) |
| MRT, h | 12.9 (3.0) | 19.6 (5.3) | 1.5 (0.18) | 20.7 (4.8) | 1.1 (0.26) |
| AUC0-∞, IU⋅h/dL | 1168 (425) | 2073 (778) | 1.9 (0.91) | 2009 (631.53) | 1.1 (0.5) |
| CL, dL/(kg⋅h) | 0.0455 (0.0217) | 0.0276 (0.0203) | 0.613 (0.28) | 0.0247 (0.00823) | 1.0 (0.27) |
| IR, (IU/dL)/(IU/kg) | 2.37 (0.536) | 2.49 0.694) | 1.1 (0.36) | 2.3 (0.64) | 1.0 (0.22) |
Mean (standard deviation [SD]) results from the 1-stage clotting assay are shown. Similar results were obtained with the chromogenic assay: T1/2 ratio for BAX 855 Initial/Advate was 1.5 (0.4622), and for BAX 855 final/initial was 1.1 (0.2906)
The 1-stage clotting assay data shown represent median (Q1; Q3) FVIII plasma levels and the nominal sampling times as indicated on the linear x axis. BAX 855 (dashed gray line) demonstrated an extended half-life compared with Advate (solid black line). The PK assessments shown were conducted in 26 subjects at their first exposure to BAX 855.
The 1-stage clotting assay data shown represent median (Q1; Q3) FVIII plasma levels and the nominal sampling times as indicated on the linear x axis. BAX 855 (dashed gray line) demonstrated an extended half-life compared with Advate (solid black line). The PK assessments shown were conducted in 26 subjects at their first exposure to BAX 855.
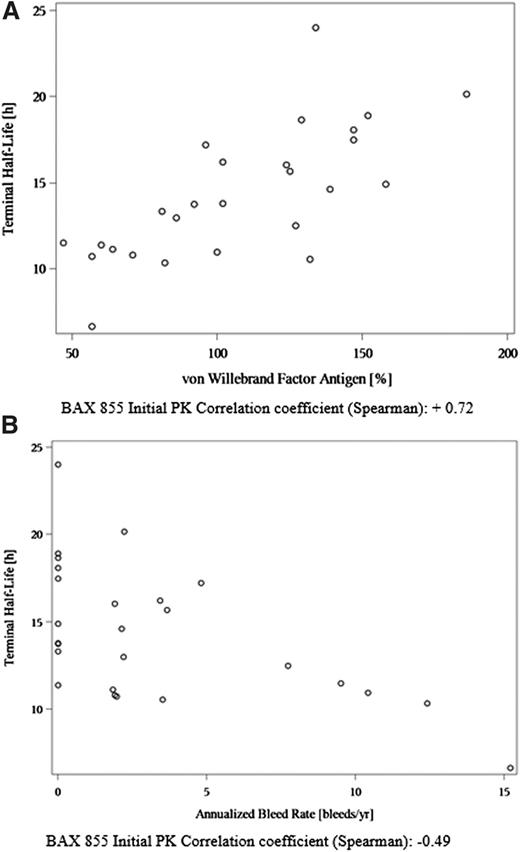
Preinfusion VWF:Ag concentration was positively correlated with T1/2 for both Advate and for BAX 855 at the initial PK and the repeat PK (Spearman’s rank correlation coefficients of +0.63, + 0.72, and + 0.35, respectively) (Figure 5), which was similar in the phase 1 study (Spearman’s rank correlation coefficients were +0.62 and +0.68 for Advate and the combined BAX 855 doses, respectively).
Scatterplots and Spearman rank correlation analysis of T1/2 are displayed. The T1/2 of BAX 855 was positively correlated to preinfusion VWF antigen plasma concentrations (A) and negatively correlated with ABR (B). The T1/2 values for these analyses were derived from PK assessments conducted in 26 subjects at their first exposure to BAX 855, using the 1-stage clotting assay. The circles represent individual subject values. An apparent outlier appears in A for a subject with a very low T1/2 and a high ABR; it is of note that no inhibitory antibodies to FVIII were detected in this subject.
Scatterplots and Spearman rank correlation analysis of T1/2 are displayed. The T1/2 of BAX 855 was positively correlated to preinfusion VWF antigen plasma concentrations (A) and negatively correlated with ABR (B). The T1/2 values for these analyses were derived from PK assessments conducted in 26 subjects at their first exposure to BAX 855, using the 1-stage clotting assay. The circles represent individual subject values. An apparent outlier appears in A for a subject with a very low T1/2 and a high ABR; it is of note that no inhibitory antibodies to FVIII were detected in this subject.
Patient-reported outcomes and quality of life
In the hierarchical test of PROs, assessed at baseline and at study completion, there were no statistically significant differences in QoL or symptoms over time identified in the prophylactic group relative to the on-demand group. However, the change in the Physical Component Score (2.67), Role Physical (4.90), Physical Functioning Score (4.21), and Social Functioning Score of the SF-36 (5.45) in the subjects receiving prophylaxis relative to subjects treated on-demand was larger than established minimally important differences for the SF-36, and therefore may be considered clinically meaningful changes.
Safety
In the phase 1 study, subjects received a single infusion of BAX 855. No subject experienced an SAE or an AE that was temporally associated with the infusion, and no subject discontinued the study after exposure because of an AE. Eight subjects experienced a total of 11 nonserious AEs, none of which was considered related to BAX 855, and all of which were consistent with the known safety profile of Advate.
In the pivotal study, a total of 171 AEs were reported in 73 (53.3%) subjects after the administration of BAX 855 for approximately 6 months. Of these, 5 were SAEs (all considered not related to BAX 855 treatment) in 5 (3.6%) subjects, including osteoarthritis, herpes zoster infection, humerus fracture, and neuroendocrine carcinoma and muscle hemorrhage. There were 7 adverse reactions in 6 subjects that were considered to have a reasonable possibility of a causal relationship with the use of BAX 855, including diarrhea, nausea, headache, and flushing, all of which are consistent with the known safety profile of Advate.
In the phase 1 study, no subjects had detectable binding antibodies with confirmed specificity to FVIII, PEG-FVIII, or PEG at any point. In the pivotal study, no subjects developed inhibitory antibodies to FVIII of 0.6 BU/mL or more, antibodies to Chinese hamster ovary proteins, or persistent binding antibodies to FVIII, PEG-FVIII, or PEG. Nine subjects had preexisting binding antibodies before exposure with BAX 855: 1 subject with anti-FVIII IgG, 6 subjects with anti-PEG-FVIII IgG, and 2 subjects with anti-PEG-FVIII IgM and anti-PEG IgM antibodies. Seven subjects who tested negative at screening developed transient IgG antibodies against FVIII (4 subjects) or PEG-FVIII (3 subjects) at 1 or 2 consecutive visits after exposure to BAX 855. Binding antibodies that were detected before exposure to BAX 855 or that transiently developed during the study could not be correlated to an impaired treatment efficacy or related AEs. No subjects who developed transient antibodies had PK analysis, and only 3 of the 9 subjects with preexisting antibodies participated in the PK analysis, so no meaningful conclusion can be drawn; however, the currently limited available data do not indicate an effect of binding antibodies on PK parameters.
Analysis of clinical chemistry, hematology, and lipid panel laboratory assessments suggest there were no definite trends toward abnormality over time, and clinically significant values could be attributed to preexisting conditions.
Discussion
This is a report of the phase 1 and pivotal clinical studies for BAX 855, a pegylated full-length rFVIII product with extended half-life designed to reduce the frequency of prophylactic infusions while maintaining hemostatic efficacy in patients with hemophilia A. These studies evaluated the safety, efficacy, PK profile, and immunogenicity of BAX 855, administered as prophylactic and on-demand treatments. The PK results from both studies confirmed an extended half-life of BAX 855 compared with Advate, demonstrating that T1/2 and MRT of BAX 855 were extended by approximately 1.4-fold and 1.5-fold, respectively. A repeat PK assessment with BAX 855 conducted after at least 50 EDs showed consistent PK parameters.
VWF:Ag levels were positively correlated with T1/2 of both Advate and BAX 855, which can be explained by the role VWF plays in protecting FVIII from premature degradation in plasma.17 Such a positive correlation between T1/2 of rFVIII and VWF levels has been previously reported.18,19 This finding suggests that the clinical management of prophylaxis in subjects with low VWF:Ag levels be monitored more closely, as these subjects might benefit from a personalized dosing regimen.20
Subjects in the prophylaxis group had a significantly reduced (90%) ABR compared with those in the on-demand group. This reduction in ABR, with a twice-weekly infusion frequency with BAX 855, is comparable to reductions in ABR reported by Valentino et al with the use of Advate for prophylaxis administered every 2 to 3 days.9 In that study, the goal of the PK-directed group was to attain a 1% FVIII trough level for Advate, which was similar to the approach taken in this study with BAX 855. This result demonstrates that BAX 855 can achieve the same degree of bleeding prevention as Advate with 1 fewer infusion per week, providing an additional treatment option for patients with hemophilia A. The majority of subjects in the prophylactic group reduced the frequency of dosing from their prestudy prophylactic treatment regimens by at least one fewer prophylactic infusion per week when using BAX 855 for prophylaxis, also supporting the use of twice-weekly infusions. The time between bleeding episodes was longer for subjects treated prophylactically with BAX 855 compared with subjects treated on-demand. Approximately 60% of patients receiving prophylaxis achieved 5 or more months between any hemorrhages, including 39.6% with zero bleeding events during the treatment period.
ABR showed a negative correlation with T1/2 of BAX 855, suggesting a shorter T1/2 is associated with a higher rate of bleeding. This finding further argues for performing PK evaluations on patients with hemophilia A to determine whether they might benefit from a personalized prophylactic dosing regimen.
Subjects in the prophylactic group reported clinically meaningful, although not statistically significant, improvements from baseline to follow-up, relative to subjects in the on-demand group and according to established minimally important differences in the Physical Component, Role Physical, Physical Functioning, and Social Functioning Scores of the SF-36 questionnaire. However, the study was not powered for this comparison. In addition to the imbalance between the sample sizes of the prophylaxis (121) and on-demand treatment (17) groups, the majority of subjects in the prophylaxis group (82.6%) were receiving prophylaxis before the study, which made demonstration of an improvement of PROs in this population difficult.
BAX 855 demonstrated efficacy in the treatment of bleeding episodes. Of all bleeding episodes treated with BAX 855, 95.9% were treated with 1 or 2 infusions, and 96.1% had a hemostatic efficacy rating of excellent or good. This is comparable to the results with Advate, in which the majority of bleeding episodes were treated with 1 or 2 infusions and the hemostatic efficacy of treatment was rated as excellent or good.9,21-23
Chemical modification with PEG is a well-established method to improve the PK profile by extending T1/2 and circulation of therapeutic proteins.13 Preclinical animal studies of BAX 855 showed extended T1/2 and clinically relevant prolonged efficacy compared with Advate without signs of toxicity or immunogenicity.14 To confirm the safety of pegylated rFVIII, safety and immunogenicity of BAX 855 were assessed in both clinical studies. There were no deaths, SAEs, or allergic reactions related to the use BAX 855 during the studies. No increased risk for previously treated patients to develop inhibitory antibodies to FVIII after treatment with BAX 855 was observed. There were no immune responses to BAX 855 with a clinical effect and no evidence of hypersensitivity reactions. No subject developed persistent binding antibodies to FVIII, PEG-FVIII, or PEG. No new safety concerns were identified in the evaluation of laboratory values and vital signs over time with the use of BAX 855. The results described in this report reflect a relatively short-term exposure (6 months) to BAX 855; however, the adverse reactions reported were consistent with the safety profile of Advate, indicating no effect of FVIII-pegylation on product safety.
In conclusion, the results of these studies provide evidence that BAX 855 is safe and efficacious for treating bleeding episodes and for prophylaxis administered twice weekly in patients with severe hemophilia A.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We recognize with gratitude the patients and investigators who participated in the studies (a complete list of investigators is available in the supplemental Data). We also thank the BAX 855 clinical study teams for operational and administrative support: Erik Bjornson, Bruce Brown, Maureen Conlan, Iraj Daizadeh, Josh Epstein, Sandor Fritsch, Frank Horling, Diane Ito, Manuela Koska, Stephan Lehr, Marzena Murawska, Said Omar, Marielle Parise, Elizabeth Schwartz, Marlies Sharkawy, Dikla Sharon, Julia Singer, Barbara Valenta-Singer, and Suzanne Wilson.
The study was funded by Baxter BioScience, which has been renamed Baxalta.
Authorship
Contribution: B.A.K., O.S., and P.C., from the pivotal study, and T.M., D.H.B., and M.S., from the phase 1 study, participated in the studies and contributed to the writing and critical review of the manuscript. B.A. oversaw the conduct of the pivotal study and analyzed and interpreted data. M.F. reviewed pivotal study data, and L.P. interpreted data and critically reviewed and contributed to the writing of the manuscript. B.E. oversaw the design of both studies and critically reviewed the manuscript. W.E. performed the sample size simulations and the statistical analyses, interpreted the results, and reviewed the manuscript. J.D.-J. interpreted data and prepared the manuscript.
Conflict-of-interest disclosure: B.A.K. received research support from Baxter, Biogen-Idec, NovoNordisk, and Octapharma and performed consultancy work for Baxter, Biogen-Idec, Bayer, NovoNordisk, Pfizer, and CSL Behring. O.S. received research funding and honoraria from Baxter Healthcare Corporation. P.C. received research support from CSL Behring, Novo Nordisk, and Pfizer and served on advisory boards for Baxter, Biogen, CSL Behring, Novo Nordisk, Pfizer, and Sobi. D.H.B. received grants from Grifols; personal fees from Pfizer and CSL Behring; grants, personal fees, and nonfinancial support from Baxter; grants and nonfinancial support from Novo-Nordisk and Biogen-Idec; personal fees and travel support from SOBI; and grants from Alnylam outside the submitted work. D.H.B. has also served on advisory boards for Pfizer, CSL Behring, and SOBI. T.M. is employed by Quintiles, received support from Baxter to conduct the study, and is supported by the National Institute for Health Research Biomedical Research Centre at Guy’s and St Thomas’ National Health Service Foundation Trust and King’s College London. M.S. received research support from Chugai, Bayer, Baxter, NovoNordisk, Pfizer, CSL-Behring, KAKETSKEN, and Biogen-Idec; performed consultancy work for Chugai Pharmaceutical; received honoraria from Chugai, Roche, Bayer, Baxter, NovoNordisk, Biogen-Idec, and Pfizer; and was member of a scientific advisory board for Chugai, Roche, Bayer, Baxter, Biogen-Idec, CSL Behring, NovoNordisk, and Pfizer. B.A., B.E., W.E., M.F., L.P., and J.D.-J. are employees of Baxalta and own stocks or shares in Baxalta/Baxter, the sponsor of the study.
Correspondence: Brigitt E. Abbuehl, Baxalta Innovations GmbH, Donau-City-Strasse 7, A-1220 Vienna, Austria; e-mail: brigitt.abbuehl@baxalta.com.