Key Points
Following vessel injury, the extravasation of plasma borne molecules continues long after hemostasis occurs.
Limiting molecular extravasation is driven by platelet accumulation and retraction, but not fibrin deposition.
Abstract
Previous studies have shown that hemostatic thrombi formed in response to penetrating injuries have a core of densely packed, fibrin-associated platelets overlaid by a shell of less-activated, loosely packed platelets. Here we asked, first, how the diverse elements of this structure combine to stem the loss of plasma-borne molecules and, second, whether antiplatelet agents and anticoagulants that perturb thrombus structure affect the re-establishment of a tight vascular seal. The studies combined high-resolution intravital microscopy with a photo-activatable fluorescent albumin marker to simultaneously track thrombus formation and protein transport following injuries to mouse cremaster muscle venules. The results show that protein loss persists after red cell loss has ceased. Blocking platelet deposition with an αIIbβ3 antagonist delays vessel sealing and increases extravascular protein accumulation, as does either inhibiting adenosine 5′-diphosphate (ADP) P2Y12 receptors or reducing integrin-dependent signaling and retraction. In contrast, sealing was unaffected by introducing hirudin to block fibrin accumulation or a Gi2α gain-of-function mutation to expand the thrombus shell. Collectively, these observations describe a novel approach for studying vessel sealing after injury in real time in vivo and show that (1) the core/shell architecture previously observed in arterioles also occurs in venules, (2) plasma leakage persists well beyond red cell escape and mature thrombus formation, (3) the most critical events for limiting plasma extravasation are the stable accumulation of platelets, ADP-dependent signaling, and the emergence of a densely packed core, not the accumulation of fibrin, and (4) drugs that affect platelet accumulation and packing can delay vessel sealing, permitting protein escape to continue.
Introduction
Recent reports show that hemostatic thrombi formed in vivo in mice or ex vivo in studies using human blood develop a heterogeneous structure1-5 in which a core of fully-activated platelets and fibrin is overlaid by a shell of less-activated platelets, forming a barrier that limits red cell loss.6 A prominent characteristic of the core is that it consists of densely packed platelets, with increased packing density driven by contraction of crosslinked platelets through αIIbβ3 integrin-dependent outside-in signaling.6-9 We have shown that the tighter packing within the thrombus core restricts molecular transport, helping to produce a region with increased local thrombin activity and greater platelet activation. Based on studies performed primarily in cremaster muscle arterioles, we proposed that this heterogeneous architecture is important for regulating the distribution of soluble agonists and, therefore, thrombus growth and core formation.6 Here we extend those observations into venules, at the same time asking, first, how the diverse elements of thrombus structure combine to prevent loss of plasma-borne molecules as well as red cells and, second, asking whether antiplatelet agents and anticoagulants that perturb thrombus structure affect the re-establishment of a tight vascular seal.
When viewed from a hydrodynamic perspective, penetrating injuries allow red cells and plasma to permeate through the injury site and accumulate in extravascular spaces, driven by the pressure differential between the vessel lumen and the surrounding tissue. Hemostatic thrombi halt both forms of loss by restricting movement within and across the thrombus microenvironment.10-15 Consistent with this role, we have previously shown that thrombi formed from human blood flowing over a collagen/tissue factor (TF) surface have a Darcy permeability approaching that of intact endothelium (κ = 2 × 10−14 cm2).5 Since plasma and platelets are the source of numerous coagulation factors,16-18 growth factors,19-22 and mediators of inflammation,23,24 transport properties at the injury site can also be viewed as regulators of wound healing and recovery. Tying function to structure, we propose that the complex architecture of hemostatic thrombi is needed in part to achieve “plasma stasis” and that this might require attributes that are not entirely the same as those required to stop red cell loss.
In this study we have tested these ideas, first by developing a novel method for quantifying plasma protein extravasation after penetrating injuries in the mouse microvasculature in real time and, second, by combining intravital microscopy with pharmacologic and genetic manipulations to resolve the contributions of platelets, fibrin, clot retraction, and thrombus architecture in vessel sealing. Our previous studies focused on the hemostatic response in arterioles. To extend that work, we studied venules as well. The results show that the core-and-shell architecture observed in arterioles is largely recapitulated in venules. The results also show that although red cell escape is halted after a few platelets are deposited in small injuries, a larger, more stringent structure is required to limit plasma protein extravasation and achieve plasma stasis. Plasma stasis proved to be highly sensitive to small changes within the platelet mass, including changes in packing density, but is less dependent on thrombin and fibrin. Plasma stasis is especially driven by adenosine 5′-diphosphate (ADP)-dependent signaling and defects in clot retraction, particularly when combined with defects in platelet accumulation, slow the restoration of a tight vascular seal.
Together with previous studies, these results show how hemostatic thrombi mature over time, gradually acquiring the attributes needed to halt the loss of plasma as well red cells until wound healing can occur. The results also show how αIIbβ3 and P2Y12 antagonists can prolong the escape of plasma-borne molecules in the microvasculature by delaying the formation of an effective vascular seal.
Materials and methods
Mice
Male mice 8 to 12 weeks of age were used in intravital experiments. C57Bl/6 mice (The Jackson Laboratory, Bar Harbor, ME) were used for experiments utilizing antiplatelet agents, and were compared with diYF mutant mice that had been backcrossed on to the C57Bl/6 background at least 7 times. DiYF mice were generated by David Phillips at Portola Pharmaceuticals (San Francisco, CA)8 and obtained with his approval from Tatiana Byzova at the Cleveland Clinic Foundation (Cleveland, OH). Gi2α(G184S) mutant mice were provided by Richard Neubig (Michigan State University). The mice used in this study were heterozygous for the mutant allele (Gnai2G184S), which we have previously showed confers a gain of function in platelets in vitro and in vivo.25 Wild-type (WT) littermates were used as controls. Mouse studies were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Caged albumin (cAlb) synthesis
As described previously,13 bovine serum albumin (BSA) (Jackson Immunological Research West Grove, PA) was labeled with 5-carboxymethoxy-2-nitrobenzyl (CMNB)-caged carboxyfluorescein, SE (5-carboxyfluorescein-bis-[5-carboxymethoxy-2-nitrobenzyl] ether, β-alanine-carboxamide, and succinimidyl ester) (Life Technologies, Frederick, MD). Briefly, the BSA was solubilized in 0.1 M sodium bicarbonate in phosphate buffered saline to a final concentration of 10 mg/mL. Concurrently, 1 mg of CMNB-caged carboxyfluorescein was solubilized in 100 μL of dimethyl sulfoxide and was thoroughly mixed with the BSA solution. The reaction was incubated for 1 hour at room temperature and passed over a 7 kDa cutoff desalting column (Thermo Scientific, Waltham, MA).
Intravital microscopy and injury generation
Mice were anesthetized with sodium pentobarbital (90 mg/kg) through an intraperitoneal injection. The jugular vein was cannulated for infusion of fluorescent markers and pharmaceutical agents. The cremaster muscle, exposed and prepared for viewing by confocal microscopy was under continuous flow of bicarbonate buffer maintained at 37°C and bubbled with 95% / 5% N2/CO2. Venules with a diameter of 30 to 50 μm were studied. Injuries were inflicted with a pulsed nitrogen dye laser (SRS NL100, 440 nm). The laser was fired until red blood cells escaped the vessel, indicating sufficient vessel wall damage.6 The area was then visualized with a BX61WI microscope (Olympus, Center Valley, PA) with a ×60 (0.9 NA) water immersion objective, and a CSU-X1 spinning disk confocal scanner (Yokogawa, Sugar Land, TX). Fluorescence imaging was performed using DPSS lasers with acousto-optic tunable filter control as the excitation source (LaserStack; Intelligent Imaging Innovations, Denver, CO). Images were captured with an Evolve EM-CCD digital camera (Photometrics, Tucson, AZ).
Platelets were visualized by anti-GPIbβ (clone Xia.C3; Emfret Analytics, Eibelstadt, Germany) or anti-CD41 (F[ab]2 fragment, clone MWReg30; BD Biosciences, San Jose, CA). Anti-CD62P (P-selectin; immunoglobulin G, clone RB40.34; BD Biosciences) was used to label degranulated platelets, and antifibrin antibody (clone 59D8; a generous gift from Dr Hartmut Weiler and Dr Rodney Camire) was used to label fibrin. All antibodies were labeled with either Alexa Fluor 568 or 647 using the Alexa Fluor Monoclonal Antibody Labeling Kit, according to the manufacturer’s instructions (Invitrogen).
Plasma protein extravasation (denoted here as leakage or L[t]) was measured by the extravasation of cAlb, which was uncaged every 15 seconds (every 15 frames at 1 frame per second) by exposure of 405 nm light and then quantified by the resulting increase in fluorescence with excitation at 488 nm (see supplemental Figure 2B, available on the Blood Web site). Thrombus formation was modified by the addition of cangrelor (a generous gift from The Medicines Company, Parsippany, NJ), hirudin (a gift from Dr Sriram Krishnaswamy, Children’s Hospital of Philadelphia), or eptifibatide (Integrilin; purchased from Schering-Plough).
In pilot studies, plasma protein extravasation was measured in both arterioles and venules. Although similar extravasation dynamics were observed in both, only venule data were used for the studies reported here because the thicker walls of the arterioles resulted in some of the leaked plasma being retained within the wall in a pseudo-aneurysm that interfered with extravasation measurements.
In vitro thrombus permeability measurements
A sidearm microfluidic chamber was used to generate thrombi ex vivo as previously described.5 Briefly, re-calcified (10 mM) citrated whole blood, from WT and diYF mice, was perfused over a collagen/TF surface to induce thrombus formation. Blood flow was maintained at a shear rate of 200 s−1, and a transthrombus pressure drop of 15 mm Hg. Platelet and fibrin deposition were monitored for 10 minutes, and then Texas Red fluorophore (Life Technologies) was infused and dynamically imaged to measure the thrombus permeability as described.5,12
Statistics
The Mann–Whitney U test was used to determine if cAlb extravasation between two different conditions was significantly different 200 seconds after injury, and the initial slope (0-60 seconds postinjury) of the cAlb extravasation curve. Student t test was used to analyze the peak/final CD41 positive area and peak P-selectin area for each condition.
Results
Thrombus formation and maturation limits plasma protein loss from the site of injury
As with arterioles,6 penetrating injuries in mouse venules evoked a non-occlusive hemostatic thrombus that differentiated into a P-selectin (+) core and a P-selectin (−) shell during the first few minutes after injury (supplemental Figure 1). To track plasma protein loss from the injury site in real time, we infused albumin conjugated with caged carboxyfluorescein (“cAlb”) prior to injury. Repeated pulses of 405 nm light were used to flash-activate cAlb that escaped into extravascular tissue planes (Figure 1A, supplemental Figure 2A-B, and supplemental Video 1). By measuring the gain in extravascular cAlb fluorescence at 15 second intervals, we were able to quantify relative cAlb extravasation (denoted here as leakage over time or (L[t]) up to 20 minutes following injury (supplemental Figure 2B-C).
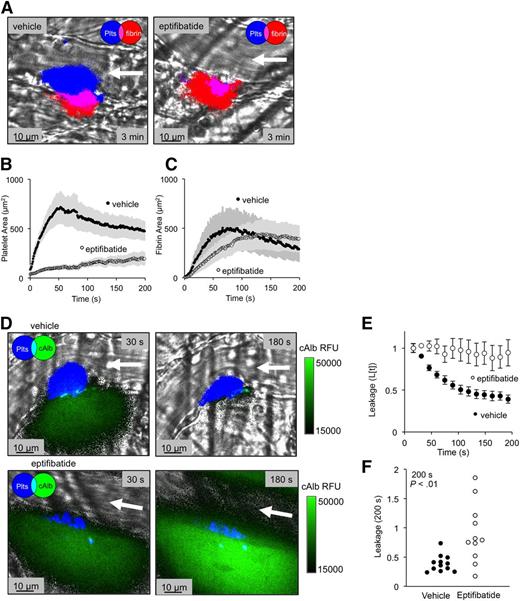
Measuring plasma protein extravasation in vivo. (A) Representative images of a thrombus formed in a mouse cremaster venule showing platelets (blue) and flash-activated cAlb (green). Each image is taken directly after an activating pulse of 405 nm light. (B) Time course of average platelet (black) and fibrin (white) area (n = 24; ± standard error of the mean [SEM]). (C) For a separate data set we tracked average platelet area (black) and P-selectin–positive area (white) (n = 23; ± SEM). (D) Time course of the combined average of relative cAlb extravasation (n = 47; ± SEM error bars too small to be visualized). (E) A dot plot of the relative cAlb extravasation for each injury at 200 seconds postinjury (n = 47). Plts, platelets; RFU, relative fluorescence units.
Measuring plasma protein extravasation in vivo. (A) Representative images of a thrombus formed in a mouse cremaster venule showing platelets (blue) and flash-activated cAlb (green). Each image is taken directly after an activating pulse of 405 nm light. (B) Time course of average platelet (black) and fibrin (white) area (n = 24; ± standard error of the mean [SEM]). (C) For a separate data set we tracked average platelet area (black) and P-selectin–positive area (white) (n = 23; ± SEM). (D) Time course of the combined average of relative cAlb extravasation (n = 47; ± SEM error bars too small to be visualized). (E) A dot plot of the relative cAlb extravasation for each injury at 200 seconds postinjury (n = 47). Plts, platelets; RFU, relative fluorescence units.
The results show an initial rapid loss of cAlb after injury, followed by a gradual decrease in extravasation over 3 minutes (Figure 1A-D). We followed cAlb concurrently with red cell loss, platelet accumulation, fibrin deposition, and P-selectin exposure, allowing us to compare the kinetics of each event. As in arterioles, we found that red cell loss from venules ended within seconds, well before peak fibrin and platelet accumulation ∼60 seconds after injury (Figure 1B-C and supplemental Video 2). cAlb extravasation continued even longer, declining over time, but not halting completely during the 20-minute time of longest observation (Figure 1D and supplemental Figure 2C). The continued decline in cAlb escape after peak platelet and fibrin accumulation is informative, suggesting that ongoing structural changes within the thrombus contribute to limiting protein losses (Figure 1B-D). Relative cAlb extravasation rates 200 seconds after injury were ∼60% less than at 15 seconds (Figure 1E and supplemental Figure 2C). Taken together, these data demonstrate that, first, in this injury model very few platelets are needed to halt red cell escape and, second, plasma protein extravasation is greatly reduced by thrombus formation, but continues well after stable thrombus formation has been achieved (supplemental Figure 2).
The contribution of platelet number to vessel sealing
The initial rapid decrease in plasma protein extravasation (∼60 seconds postinjury) coincided with initial platelet deposition and fibrin accumulation. To determine how each of these events contributes to vessel sealing, we infused the integrin αIIbβ3 antagonist, eptifibatide (20 mg/kg)26 to inhibit platelet cohesion (Figure 2A-C). Eptifibatide caused a ∼70% decrease in peak platelet area (P < .001) with a small decrease in the kinetics of fibrin accumulation. The decrease in platelet accumulation had no impact on peak fibrin formation (P = .79) (Figure 2A-C) or on cessation of red cell loss (not shown), but the ability of the thrombi to stop extravasation of cAlb was greatly reduced (Figure 2D-F and supplemental Videos 1 and 3). When measured 200 seconds after injury, we found a large variation in the ability of eptifibatide-treated thrombi to limit cAlb extravasation. Several eptifibatide-treated thrombi showed increased cAlb extravasation at 200 seconds compared with 15 seconds, and several more showed only minor reductions in extravasation resulting in an average extravasation rate significantly larger than the vehicle-treated thrombi (Figure 2F). This large variation may be due to variations in the extent of injury, with smaller injuries able to form a seal with fewer platelets and larger injuries leaking at a higher rate.
Inhibition of αIIbβ3 integrin reduces platelet accumulation and vessel sealing. (A) Representative images of thrombi formed in the presence of either vehicle (left) or eptifibatide (right) (20 mg/kg), showing both platelets (blue) and fibrin (red) 3 minutes postinjury. (B) Average platelet area and (C) fibrin area for both the vehicle-treated (black) and eptifibatide-treated (white) thrombi (± SEM). (D) Representative images of cAlb extravasation (green) at both 30 and 180 seconds postinjury for vehicle (top) and eptifibatide treated (bottom) thrombi (platelets are denoted in blue). (E) Time course and (F) dot plot of relative cAlb extravasation for vehicle (black) and eptifibatide (white) treated thrombi (vehicle n = 12, eptifibatide n = 11; ± SEM).
Inhibition of αIIbβ3 integrin reduces platelet accumulation and vessel sealing. (A) Representative images of thrombi formed in the presence of either vehicle (left) or eptifibatide (right) (20 mg/kg), showing both platelets (blue) and fibrin (red) 3 minutes postinjury. (B) Average platelet area and (C) fibrin area for both the vehicle-treated (black) and eptifibatide-treated (white) thrombi (± SEM). (D) Representative images of cAlb extravasation (green) at both 30 and 180 seconds postinjury for vehicle (top) and eptifibatide treated (bottom) thrombi (platelets are denoted in blue). (E) Time course and (F) dot plot of relative cAlb extravasation for vehicle (black) and eptifibatide (white) treated thrombi (vehicle n = 12, eptifibatide n = 11; ± SEM).
Given these results, which highlight the role of platelets in achieving a temporary vascular seal, we next tested whether an increase in thrombus size would accelerate the decline in cAlb extravasation rates or result in formation of an even tighter seal. To do this, we studied mice bearing a germ line substitution (G184S) in the α subunit of Gi2 that blocks negative feedback by regulators of G-protein signaling (RGS) proteins, producing a gain of function in platelets activated by agonists such as ADP.6 As in arterioles,6 laser injury to venules induced 72% larger thrombi in the Gi2α(+/G184S) mice than in paired controls, primarily due to the expansion of the P-selectin (−) shell (supplemental Figure 3A-B). However, the dynamics of vessel sealing were unaffected, implying that normal sized thrombi reflect the limit to how much platelets can restrict protein loss in this injury model, at least in terms of the shell region (supplemental Figure 3C).
Platelet retraction contributes to vessel sealing
Our previous studies demonstrated the importance of platelet packing density in limiting intrathrombus solute transport and showed that platelet-dependent clot retraction was an important determinant of packing density.7 To test the hypothesis that platelet retraction helps to limit plasma protein loss after injury, we measured cAlb extravasation in mice in which two critical tyrosines in the cytoplasmic domain of the β subunit of integrin αIIbβ3 were replaced with phenylalanine residues (denoted diYF). The platelets from these mice show normal integrin activation, but have a defect in outside-in signaling that leads to delayed clot retraction in vitro and reduced thrombus consolidation in vivo.7,8
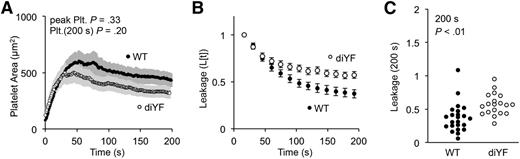
Consistent with our prior studies in arterioles, we observed no significant defect in either the kinetics of platelet accumulation or the total area of the platelet mass in the diYF mice (Figure 3A). For the first 60 seconds (the period of greatest platelet accumulation), there was also no difference between diYF and control mice in their ability to slow the rate of cAlb extravasation (Figure 3B). After 60 seconds, however, cAlb loss in the diYF mice remained elevated compared with the controls. This difference was smaller than that observed with eptifibatide (compare Figure 3B with 2E). It suggests that after initial platelet recruitment, platelet retraction drives intrathrombus remodeling to further seal against plasma losses at the injury site. The retraction defect in the diYF mice resulted in a 54% increase in mean relative cAlb extravasation compared with controls at 200 seconds postinjury (Figure 3C).
Outside-in signaling drives platelet retraction and vessel sealing. (A) The average platelet area for WT (black) and diYF thrombi (white) (± SEM). (B) Time course (mean ± SEM) and (C) dot plot at 200 seconds postinjury of cAlb extravasation for WT (black) and diYF (white) thrombi (WT n = 24, diYF n = 19).
Outside-in signaling drives platelet retraction and vessel sealing. (A) The average platelet area for WT (black) and diYF thrombi (white) (± SEM). (B) Time course (mean ± SEM) and (C) dot plot at 200 seconds postinjury of cAlb extravasation for WT (black) and diYF (white) thrombi (WT n = 24, diYF n = 19).
These differences were not dependent on vessel wall structures: a similar difference was observed in Darcy permeability measurements performed ex vivo in a microfluidics device using WT and diYF mouse blood. The ex vivo studies show an increase in permeability in diYF thrombi without a corresponding change in thrombus size (supplemental Figure 4A-C). We also found no correlation between platelet area and cAlb extravasation in either the WT or diYF thrombi formed in vivo, suggesting that retraction and not experiment to experiment variations in thrombus size are responsible for the increased cAlb extravasation observed in the diYF injuries (supplemental Figure 4).
Taken together, these results suggest that the initial phase of vessel sealing is driven by the rapid accumulation of platelets, whereas continued sealing is driven in part by the same integrin αIIbβ3-mediated retraction that increases platelet packing density and decreases thrombus permeability. Thus, once platelets accumulate, cAlb extravasation is sensitive to small changes in the intrathrombus structure.
The role of fibrin accumulation, ADP-dependent signaling, and thrombus architecture in restricting plasma protein loss
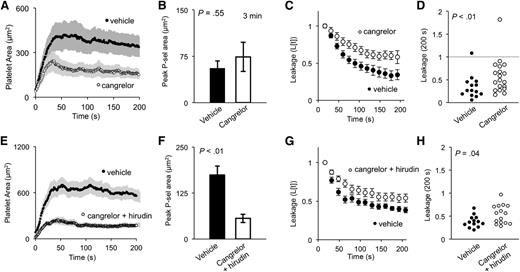
In our prior studies on arterioles, we found that thrombin activity and fibrin accumulation are restricted to the thrombus core.6,27 The addition of a direct thrombin antagonist, such as hirudin, abolished fibrin accumulation and decreased both overall thrombus size and the size of the core (defined in those studies as the P-selectin [+] region). In the present studies, we found that hirudin also decreases thrombus size in venules, but is not quite sufficient by itself to significantly reduce platelet activation measured by P-selectin exposure (Figure 4). Fibrin accumulation was abolished (Figure 4C), but the ability to form a seal was essentially unaffected (Figure 4E-F). This finding, along with the findings of the eptifibatide studies, suggests that fibrin accumulation plays little or no role in preventing the extravasation of plasma proteins in this injury model.
Thrombin inhibition decreases fibrin accumulation and thrombus size, but not vessel sealing. (A) Representative images of thrombi formed either in the presence of vehicle (left) or thrombin inhibitor hirudin (right) (∼0.7 mg/g) showing platelets (blue) and P-selectin (red) 3 minutes postinjury. (B) Quantification of average platelet area, (C) peak fibrin area, and (D) peak P-selectin area for both vehicle-treated (black) and hirudin-treated (white) thrombi (± SEM). (E) Time course of average cAlb extravasation (± SEM) and (F) dot plot of cAlb extravasation at 200 seconds for both vehicle-treated (black) and hirudin-treated (white) thrombi (vehicle n = 17, hirudin n = 18). P-sel, P-selectin.
Thrombin inhibition decreases fibrin accumulation and thrombus size, but not vessel sealing. (A) Representative images of thrombi formed either in the presence of vehicle (left) or thrombin inhibitor hirudin (right) (∼0.7 mg/g) showing platelets (blue) and P-selectin (red) 3 minutes postinjury. (B) Quantification of average platelet area, (C) peak fibrin area, and (D) peak P-selectin area for both vehicle-treated (black) and hirudin-treated (white) thrombi (± SEM). (E) Time course of average cAlb extravasation (± SEM) and (F) dot plot of cAlb extravasation at 200 seconds for both vehicle-treated (black) and hirudin-treated (white) thrombi (vehicle n = 17, hirudin n = 18). P-sel, P-selectin.
The results with hirudin contrast with those obtained with the direct-acting P2Y12 antagonist, cangrelor. Like hirudin, cangrelor caused a decrease in overall thrombus size which, in this case, was largely due to a reduction in the size of the P-selectin (−) shell (Figure 5A-B). However, unlike hirudin, cangrelor caused a significant increase in plasma protein extravasation and a delay in sealing (Figure 5C-D). Combining hirudin and cangrelor had an effect on extravasation that was similar to adding cangrelor alone, despite having an even greater effect on platelet deposition and P-selectin exposure (Figure 5E-H).
ADP drives fully competent core formation and shell recruitment. (A) Quantification of average platelet area, and (B) peak P-selectin–positive area of vehicle (black) and cangrelor-treated (white) thrombi. (C) Average cAlb extravasation time course and (D) dot plot of relative cAlb extravasation at 200 seconds postinjury. (A-D) Vehicle n = 15, cangrelor n = 20; ± SEM). (E) The average platelet area, and (F) peak P-selectin–positive area for vehicle (black) and cangrelor plus hirudin-treated (white) thrombi (± SEM). (G) Time course of relative cAlb extravasation, and (H) dot plot of cAlb extravasation at 200 seconds postinjury. (E-H) Vehicle, black n = 13; cangrelor plus hirudin, white n = 15.
ADP drives fully competent core formation and shell recruitment. (A) Quantification of average platelet area, and (B) peak P-selectin–positive area of vehicle (black) and cangrelor-treated (white) thrombi. (C) Average cAlb extravasation time course and (D) dot plot of relative cAlb extravasation at 200 seconds postinjury. (A-D) Vehicle n = 15, cangrelor n = 20; ± SEM). (E) The average platelet area, and (F) peak P-selectin–positive area for vehicle (black) and cangrelor plus hirudin-treated (white) thrombi (± SEM). (G) Time course of relative cAlb extravasation, and (H) dot plot of cAlb extravasation at 200 seconds postinjury. (E-H) Vehicle, black n = 13; cangrelor plus hirudin, white n = 15.
These results show that ADP is critical for re-establishing vessel sealing once platelets have begun to accumulate. This is consistent with previous observations that in human thrombi, ADP and thromboxane A2 are the major drivers of retraction in the absence of thrombin.12 The results also suggest that P-selectin exposure, although informative about α-granule exocytosis, is not directly tied to the formation of a tight vascular seal, thereby strengthening the conclusion that other properties of the thrombus core, such as clot retraction and packing density, need to be considered as well.
Plasma protein accumulation in the extravascular space
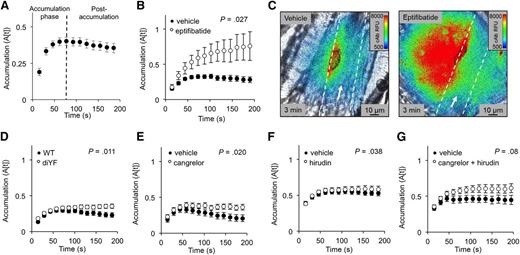
Blood-borne proteins accumulate in the extravascular space when the rate of delivery (extravasation) exceeds the rate of dispersion into the surrounding tissue. To measure the dispersion rate, we observed the rate of cAlb fluorescence decay (ΔF[decay]) in the extravascular tissue near the injury site between fluorescence-activating pulses of 405 nm light (supplemental Figure 5A-B). We found that the cAlb dispersion rate was greatest initially, peaking 60 seconds after injury, which is approximately the same time that peak platelet accumulation is achieved (compare supplemental Figure 5C with earlier figures). Despite the high initial cAlb dispersion rate, extravasation greatly exceeded dispersion during the first 60 to 75 seconds postinjury, resulting in substantial cAlb accumulation (the accumulation phase indicated in Figure 6A). However, once sufficient thrombus growth had occurred, the rate of extravasation dropped below the rate of dispersion, leading to a gradual but consistent decline in fluorescent extravascular cAlb (Figure 6A).
cAlb accumulation in the extravascular space. (A) cAlb accumulation was measured by the relative fluorescence remaining 15 seconds after each activating light pulse for 200 seconds (n = 47, ± SEM). (B) cAlb accumulation was measured for eptifibatide-treated thrombi (white), and vehicle-treated thrombi (black) (vehicle n = 12, eptifibatide n = 11; ± SEM). (C) Representative pseudo-colored images of cAlb intensity 3 minutes postinjury in vehicle-treated (left) and eptifibatide-treated (right) mice. The vessel wall is indicated by the dashed white line and platelets are outlined in black. (D) cAlb accumulation for diYF and WT thrombi, (E) cangrelor-treated, (F) hirudin-treated, and (G) cangrelor/hirudin-treated vs vehicle-treated thrombi (all n values the same as previously reported for the respective data set).
cAlb accumulation in the extravascular space. (A) cAlb accumulation was measured by the relative fluorescence remaining 15 seconds after each activating light pulse for 200 seconds (n = 47, ± SEM). (B) cAlb accumulation was measured for eptifibatide-treated thrombi (white), and vehicle-treated thrombi (black) (vehicle n = 12, eptifibatide n = 11; ± SEM). (C) Representative pseudo-colored images of cAlb intensity 3 minutes postinjury in vehicle-treated (left) and eptifibatide-treated (right) mice. The vessel wall is indicated by the dashed white line and platelets are outlined in black. (D) cAlb accumulation for diYF and WT thrombi, (E) cangrelor-treated, (F) hirudin-treated, and (G) cangrelor/hirudin-treated vs vehicle-treated thrombi (all n values the same as previously reported for the respective data set).
Perturbations in thrombus structure that impacted cAlb extravasation also affected cAlb accumulation. For example, when eptifibatide was present, cAlb accumulation increased (Figure 6B) because there was an increase in cAlb extravasation (Figure 2E) without a corresponding increase in cAlb dispersion (ΔF[decay]) (supplemental Figure 5D). This led to a continuously increasing gradient of cAlb in the tissue surrounding the injury site, whereas vehicle-treated thrombi had shrinking cAlb gradients by 200 seconds postinjury (Figure 6B-C). Thrombi formed in diYF mice also allowed for an increase in extravascular cAlb accumulation (Figure 6D), as did thrombi formed in the presence of cangrelor (Figure 6E), although these differences were small compare with eptifibatide. Consistent with the hypothesis that cAlb accumulation is primarily driven by extravasation rates, we observed no effect of hirudin alone on cAlb accumulation (Figure 6F). The combined treatment of cangrelor and hirudin did not show a statistically significant difference in accumulation despite having a significant increase in extravasation rates (Figure 6G).
Taken together, these results demonstrate that hemostatic thrombi are able to achieve a level of vessel sealing that limits plasma protein extravasation to rates less than the rate at which plasma protein dissipates into the surrounding tissue, preventing the continued concentration of plasma proteins in the surrounding tissue. Thus, small changes in the structure of the thrombus that impair vessel sealing can produce a larger than normal gradient of potentially bioactive molecules at the site of injury.
Discussion
Although it has been well established that soluble plasma-borne and platelet-derived molecules play a role in postinjury inflammation and wound healing,19-24,28 it is less clear how the delivery of these molecules is constrained following injury, especially during the period before the integrity of the endothelial monolayer is fully restored. Computational10,15,29 and experimental7,11-13,30 studies show that thrombi are capable of acting as molecular sieves, altering the transport of proteins and small molecules through dense platelet packing and fibrin formation. Here, we tested the idea that diverse properties of thrombus structure regulate protein extravasation, just as we have previously shown that they contribute to the local accumulation of thrombin and ADP, the deposition of fibrin, and the cessation of red cell loss. To investigate this hypothesis, we designed a novel sensor able to measure plasma protein extravasation in real time, allowing us to correlate changes in plasma protein transport and intrathrombus structure. The sensor is based on albumin, which is freely mobile in plasma. How other proteins behave under the same conditions remains to be determined, especially if they differ drastically in their hydrodynamic properties or have binding sites within the thrombus. However, we anticipate that binding to the platelet surface or to fibrin will primarily affect retention within the thrombus and that albumin will prove to be predictive of general transport behavior. We found that following small penetrating injuries in the microcirculation, hemostatic thrombus formation significantly reduces the rate of plasma protein extravasation, just as it brings an end to red cell escape. However, we found that plasma stasis lags well behind hemostasis, an observation that suggests that maintaining molecular exchange between the circulating blood and surrounding tissue may be beneficial, or at least not harmful, for achieving optimal posthemostatic responses.
In addition to providing information about the kinetics of vascular sealing, our results demonstrate that platelets, not fibrin, are primarily responsible for limiting the loss of soluble proteins. Data obtained with inhibitors of platelet activation and cohesion, and with the retraction-deficient diYF mice show that both platelet accumulation and retraction are involved. However, there appears to be a limit to how much platelet accumulation alone can do. Thus, although considerably larger thrombi formed in Gi2α(G184S) mice, we found no corresponding increase in the rate of vessel sealing. The inability of fibrin to significantly block plasma protein extravasation is consistent with observations in human blood, demonstrating the limited effect of fibrin on clot permeability.5,12 Together, these observations demonstrate that platelet accumulation and organization are the driving events for resisting molecular permeation into the extravascular space.
If the ability to restrict plasma protein extravasation is defined by the size and structure of the thrombus microenvironment, then the differential effects of the inhibitors that we observed are informative in examining how specific pathways shape that microenvironment. We found that both hirudin and cangrelor decrease thrombus size to a similar extent. However, hirudin had no effect on plasma protein extravasation, whereas cangrelor induced a significant increase in plasma protein extravasation. This suggests that ADP has a greater role than thrombin for establishing the changes in thrombus structure that are important for limiting protein extravasation. These results also confirm that fibrin accumulation, which was abolished by hirudin, contributes little to preventing extravasation, at least in the acute setting.
Notably, although having a greater impact on overall platelet accumulation, the combination of cangrelor and hirudin had no greater impact than cangrelor alone on plasma protein extravasation or further delay vessel sealing. Therefore, we suggest that the impact of ADP receptor inhibition on the intrathrombus microenvironment is likely limited to the region immediately adjacent to the injury site. Thus, further decreasing platelet deposition in the case of combined cangrelor-hirudin treatment does not increase plasma protein extravasation. Interestingly, the combined cangrelor-hirudin treatment also significantly decreased P-selectin exposure. However, the extent of P-selectin exposure, which we have previously used to define the thrombus core region, did not correlate with plasma protein extravasation: blocking P2Y12 receptors alone has no effect on P-selectin expression in either arterioles (our previous studies6 ) or venules (the present data), but clearly affects transport. Thus, α-granule exocytosis and vessel sealing are not irrevocably linked.
Finally, our results show that thrombus formation is capable of limiting plasma protein extravasation sufficiently to prevent the continuous accumulation of plasma borne protein within the local tissue. Because extravasated cAlb dispersed into surrounding tissues at a relatively steady rate, accumulation was primarily dependent upon the delivery rate of cAlb through the thrombus. Directly after vessel injury, rapid extravasation produced local cAlb accumulation until progressive vessel sealing slowed the rate of extravasation below the rate of dispersion (Figure 7). Within this sequence of events, platelet retraction drives the transition from plasma protein accumulation to dispersion.
Model of hemostasis in mouse cremaster venules. A model depicting the rate of plasma protein extravasation (L[t]) and dispersion (D[t]) over time, and the resulting impact on the gradient of plasma proteins in the extravascular space.
Model of hemostasis in mouse cremaster venules. A model depicting the rate of plasma protein extravasation (L[t]) and dispersion (D[t]) over time, and the resulting impact on the gradient of plasma proteins in the extravascular space.
In conclusion, our study demonstrates a novel method for measuring protein transport into the extravascular space and evaluating thrombus integrity in vivo. The results show that the hemostatic response produces a core-and-shell structure in venules that resembles the response in arterioles. We have previously suggested that the core-and-shell architecture is needed in part to allow local differential accumulation of platelet agonists.7,10,13 The results obtained here refine our understanding of the implications of thrombus structure, showing that formation of a temporary barrier against plasma protein escape requires greater platelet accumulation, ADP-dependent signaling, and increased platelet packing density, thus providing another reason for the complex architecture observed in fully hemostatic thrombi. The vascular seal is less dependent on thrombin in this setting and appears not to require fibrin accumulation. It is interesting to speculate on the extent to which our microcirculation observations apply to the macrocirculation, where conditions are considerably different. To begin to address these differences, we have recently begun studies on hemostatic thrombus formation in the femoral artery, using a modified version of methods described here. In preliminary studies, we have observed that although there are differences in thrombus size and structure, the core-and-shell architecture observed in arterioles and venules also occurs in the femoral artery.31 Others have examined these issues as well. In a notable study, Getz et al have used a different approach than the one we employed, making repeated laser injuries to exposed saphenous veins and measuring platelet accumulation and fibrin deposition, but not protein transport or extravasation.32 They also found that hemostasis was achieved before accumulation of platelets was complete and that fibrin deposition lags behind platelet accumulation. A defect in integrin activation in platelet function delayed the time until hemostasis was achieved, whereas impaired thrombin generation and fibrin accumulation in low TF and factor IX-deficient mice had relatively little effect. Finally, although not directly examined here, we assume that the delivery of soluble platelet-derived molecules, including growth factors and cytokines, is subject to the same constraints as the delivery of plasma proteins. Finally, the results obtained in the presence of members of two widely used classes of antiplatelet agents (P2Y12 and αIIbβ3 antagonists) show how these drugs can prolong the escape of plasma-borne molecules by delaying the formation of an effective vascular seal.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (NIH), National Heart, Lung and Blood Institute (NHLBI) (P01 HL40387 and P01 HL112722 to T.J.S. and L.F.B., and R01 HL103419 to S.L.D. and L.F.B.) and the American Heart Association (11SDG5720011 to T.J.S.). J.D.W. was supported by predoctoral fellowships from the American Heart Association (14PRE19560005) and the NIH NHLBI (T32 HL074398).
Authorship
Contribution: J.D.W. designed and conducted the experiments, analyzed data, and wrote the manuscript; R.W.M. and J.P.T. conducted experiments and analyzed data; and T.J.S., S.L.D., and L.F.B. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lawrence F. Brass, Department of Medicine, University of Pennsylvania, 421 Curie Blvd, Room 815, BRB-2, Philadelphia, PA 19104; e-mail: brass@mail.med.upenn.edu.

![Figure 1. Measuring plasma protein extravasation in vivo. (A) Representative images of a thrombus formed in a mouse cremaster venule showing platelets (blue) and flash-activated cAlb (green). Each image is taken directly after an activating pulse of 405 nm light. (B) Time course of average platelet (black) and fibrin (white) area (n = 24; ± standard error of the mean [SEM]). (C) For a separate data set we tracked average platelet area (black) and P-selectin–positive area (white) (n = 23; ± SEM). (D) Time course of the combined average of relative cAlb extravasation (n = 47; ± SEM error bars too small to be visualized). (E) A dot plot of the relative cAlb extravasation for each injury at 200 seconds postinjury (n = 47). Plts, platelets; RFU, relative fluorescence units.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/12/10.1182_blood-2015-09-672188/5/m_1598f1.jpeg?Expires=1771041889&Signature=h58t1~xsZcqMbOx~s0A0oeyE4I6eLWX8E81eFSzYPNIKGsIF~KvaGi8K0G6Q8sc3xAZgacuHBjDqroUjzB6gbeqmQpae7lyGPtrVV5dYXh~I5Z8TsIKFRD12Fk-I4QTRLAcsZzpJ7dD5nHzyxhtZtH~2HKDM0ct7Z73dusr2k0c3OfGIveyrgIMyBeaPP3m9MmxqxO3pqs7QXVJG0RE~jqF~qgq~jLgwoB-I~drX7gqdC~QZoaeIqwcV64nwitHWmuR4m6po4PS4VWXcLhUciiph6bcmD69dJITBXYu4wMG0Oog7z80zwT8KG6IJ~0LLssDDpKejDSrriIQOPqWrhg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. Model of hemostasis in mouse cremaster venules. A model depicting the rate of plasma protein extravasation (L[t]) and dispersion (D[t]) over time, and the resulting impact on the gradient of plasma proteins in the extravascular space.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/12/10.1182_blood-2015-09-672188/5/m_1598f7.jpeg?Expires=1771041889&Signature=VFsGLjFsvBDMy616KtKofUU1vahut~S0~E0tx97aU1LCndtGAh6CTsJpHb43VRSJzzrx1CdZD97Y3m5ByuMASqUb1CIcc4uC-N7b8SPggk55ggbVy94L92BH8JrJL8~jmUQ~yuPmeQjC7Go05qy-aLxAhvotiMJh9YGNjcaX7vloheCzW-O0ol2n2qOLfwt7obLFl1PSc9jbHwVhIefVKFM2PdCG9RgyuAKCLC74WMYCAYALqQFvPMSn0HwX0B27S5eQHYxm4sCMTaZKVxhT5I0KUuuNOj1Kv37Nx-vYhdYpWrUnwoFeZ~Ux88cAMhM7TPaTFK2uOhQFpk4MVSRBUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal