In this issue of Blood, Rowley et al report that noncoding RNAs precisely regulate the messenger RNA (mRNA) profile in platelets. Interfering in this process using genetically engineered mice affects hemostatic and thrombotic functions of platelets.1
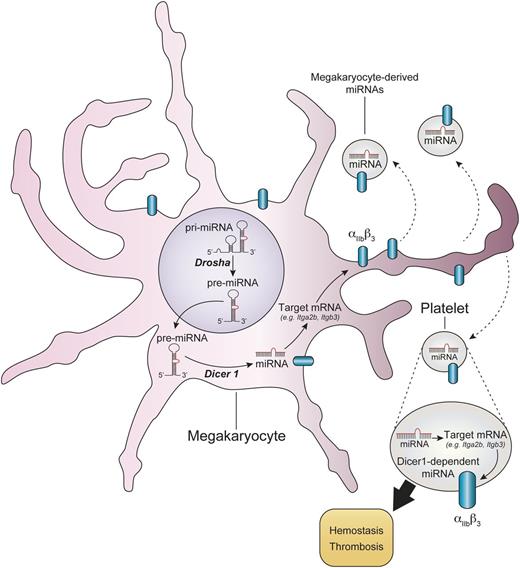
Dicer1-dependent generation of miRNAs modulate mRNA expression and functions of platelets. MicroRNA precursors (pre-miRNA) in the megakaryocyte nucleus are processed by Drosha and are then exported in the cytoplasm, where they undergo further trimming by Dicer1. Upon recognition of their mRNA targets, functional miRNAs trigger mRNA degradation or translational repression. Dicer1-dependent miRNAs modulate mRNA profiles in platelets, such as the mRNAs coding for Itgb3 (β3) and Itgb2 (αIIb). The ablation of Dicer1 in platelets leads to overexpression of αIIbβ3 protein at the platelet surface and heightened platelet reactivity. The modulation of the platelet mRNA repertoire by microRNAs can occur in the megakaryocyte and might potentially also take place in platelets once in the blood circulation or during their storage. pri-miRNA, primary miRNA.
Dicer1-dependent generation of miRNAs modulate mRNA expression and functions of platelets. MicroRNA precursors (pre-miRNA) in the megakaryocyte nucleus are processed by Drosha and are then exported in the cytoplasm, where they undergo further trimming by Dicer1. Upon recognition of their mRNA targets, functional miRNAs trigger mRNA degradation or translational repression. Dicer1-dependent miRNAs modulate mRNA profiles in platelets, such as the mRNAs coding for Itgb3 (β3) and Itgb2 (αIIb). The ablation of Dicer1 in platelets leads to overexpression of αIIbβ3 protein at the platelet surface and heightened platelet reactivity. The modulation of the platelet mRNA repertoire by microRNAs can occur in the megakaryocyte and might potentially also take place in platelets once in the blood circulation or during their storage. pri-miRNA, primary miRNA.
MicroRNAs (miRNAs) are a form of noncoding RNAs, the predominant genetic output in mammals. MicroRNAs are small (∼22 nt) single-stranded sequences generated by sequential steps of enzymatic trimming activities on double-stranded miRNA precursors. These reactions occur in the nucleus through the activities of an enzyme called Drosha, and by the action of Dicer1 in the cytosol. On recognition of their mRNA targets, miRNAs induce degradation or prevent translation of the mRNA. Thus, far from being considered junk, miRNAs act as a dimmer switch and fine-tune gene expression. Consistent with their critical role in the control of cellular functions, abrogation of miRNA biogenesis is lethal during embryonic development in mice.
Although they are anucleate, platelets are now recognized as containing mRNAs and miRNAs, the most abundant form of noncoding RNA present in platelets.2 The mRNA and miRNA repertoire mainly originates from megakaryocytes, and correlative studies suggest that miRNAs in platelets regulate platelet reactivity.3-7 To formally assess the contribution of Dicer1 and the role of miRNA in platelet functions, the authors used a conditional deletion system to delete Dicer1 in megakaryocytes and in platelets. Although no developmental or age-related abnormalities were observed in these mice, Rowley et al found that deletion of Dicer1 led to consistent reduction of miRNA expression in platelets, thus confirming the role of Dicer1 in maturation of miRNA in megakaryocytes and platelets. Although Drosha was also implicated, because its genetic ablation reduced miRNA in platelets, the combined loss of Dicer1 and Drosha did not reveal changes not seen in either of the single knockouts.
To verify whether miRNAs regulate posttranscriptional gene expression in platelets, the authors performed an RNA-seq analysis of the complete set of mRNAs contained in platelets expressing or lacking Dicer1. The targets that were the most substantially influenced by reduction of miRNAs included Itgb3 (β3) and Itgb2 (αIIb), followed by mRNAs coding for various cytoskeleton components. Considering the key roles of αIIbβ3 in platelets, the authors verified its protein expression on platelets. They confirmed that αIIbβ3 is more abundant on the platelet surface in the absence of Dicer1. Thus, ablation of Dicer1 leads to reduction of miRNA expression, thereby permitting the overexpression of their mRNA targets in platelets.
The demonstration of convincing changes, even at molecular levels, in Dicer1-null platelets was by itself a tour de force for several reasons. As previously mentioned, genetic ablation of Dicer1 in the whole mouse is lethal. Furthermore, miRNAs play essential roles in megakaryopoiesis and platelet generation, and interfering in miRNA biogenesis at a too-early stage of megakaryocyte maturation could have led to a complete loss of platelets.3,8 For their study, the authors used mice harboring a floxed allele of Dicer1 that they crossed with animals expressing Cre recombinase controlled by the platelet factor 4 (Pf4) promoter to generate the Dicer1-null platelets. Consequently, the Dicer1 gene underwent truncation in megakaryocytes at a later stage (ie, on PF4-promoter activation), thus leaving a considerable pool of miRNAs in megakaryocytes, even in the absence of Dicer1. The reduction, rather than the complete ablation, of miRNA expression in the absence of Dicer1 suggests that the miRNAs are primarily produced before PF4 activation, and it also highlights the stability of miRNA in maturing megakaryocytes and platelets.
Next, the authors explored the role of miRNAs in platelet function, paying particular attention to known roles of αIIbβ3. In the first set of experiments, they logically verified uptake of fibrinogen, which is internalized in platelets dependent on αIIbβ3 in vivo. They found that in the absence of Dicer1, platelets were more efficient at storing fibrinogen, consistent with the overexpression of αIIbβ3. Importantly, although mice lacking Dicer1 in platelets displayed modest thrombocytopenia, bleeding time was markedly reduced, further pointing to a role of miRNAs in controlling platelet activation. Then, using a model of collagen/epinephrine-induced pulmonary embolism, the authors clearly established the key role of platelet miRNAs in thrombosis. Although these experiments do not rule out the potential contribution of Dicer1-independent miRNAs, these results demonstrate that miRNAs derived from Dicer1 tightly regulate platelet reactivity (see figure).
The findings in this study have repercussions for understanding platelet functions in a multitude of ways. MicroRNAs in platelets can be encapsulated in small membrane vesicles, called “microparticles.” Because platelet-derived microparticles mediate transfer of their cargo between platelets and other cells, such as endothelial cells and leukocytes,7,9 this suggests that the (dys)regulation of miRNA content in platelets might also affect other cellular lineages. Furthermore, these observations are supported by previous studies on human platelets that miRNA content varies in different disease states and phenotypes, such as race, and correlates with platelet reactivity.4-7 MicroRNAs may also regulate platelet functions in contexts other than thrombosis, such as immunity. Hence, miR-148a regulates platelet signaling induced by immune complexes in mice.10 In sum, in addition to the potential of platelet miRNAs as biomarkers in pathogenesis, this study establishes that, generated via Dicer1, they define the hemostatic and thrombotic functions of platelets.
Conflict-of-interest disclosure: The authors declare no competing financial interests.