Abstract
Hypercholesterolemia is a risk factor for atherothrombotic disease, largely attributed to its impact on atherosclerotic lesional cells such as macrophages. Platelets are involved in immunity and inflammation and impact atherogenesis, primarily by modulating immune and inflammatory effector cells. There is evidence that hypercholesterolemia increases the risk of atherosclerosis and thrombosis by modulating platelet biogenesis and activity. This review highlights recent findings on the impact of aberrant cholesterol metabolism on platelet biogenesis and activity and their relevance in atherosclerosis and thrombosis.
Platelets and atherosclerosis
Platelets are best known for their roles in hemostasis and thrombosis. However, there is an increasing appreciation of the critical roles of platelets in immunity and inflammation in both health and disease.1-3 Atherosclerosis is a lipid-driven chronic inflammatory disease that involves localized recruitment of myeloid and immune cells (including neutrophils, monocytes, and lymphocytes) to large and medium-sized arteries.4,5 Platelets appear to play a key role in recruitment of these inflammatory effector cells.6 Activated platelets interact with endothelial cells of inflamed or atherosclerotic arteries and deposit platelet-derived cytokines such as chemokine (C-C motif) ligand 5 (CCL5) or chemokine (C-X-C motif) ligand 4 (CXCL4) onto the surface of endothelial cells, facilitating recruitment of leukocytes into the lesions.7 Genetic deficiency of CCL5 and CXCL4 or pharmacologic disruption of the functional heteromerization between CCL5 and CXCL4 decreases atherogenesis in animal models.7 Activated platelets form aggregates with neutrophils and monocytes, and the ensuing cross talk between platelets and leukocytes also plays a key role in inflammatory cytokine production, leukotriene biosynthesis, and reactive oxygen species production,8,9 with proinflammatory consequences in the vasculature. Platelet-leukocyte aggregates (PLAs) are an independent risk factor for atherothrombotic disease10-12 and promote atherogenesis in mouse models6,13 (Figure 1). An elegant recent study from Sreeramkumar et al demonstrated that neutrophils bound to endothelium scan the bloodstream for activated platelets, leading to neutrophil polarization and formation of PLAs and facilitating neutrophil migration into inflamed blood vessels.8 This interaction of platelets with leukocytes is initiated by the binding of platelet P-selectin to P-selectin ligand, which localizes to the uropod (tail) of neutrophils as they bind to endothelium. Disruption of platelet/leukocyte interactions via genetic deficiency of P-selectin13 or by anti-P-selectin–blocking antibodies decreases leukocyte recruitment and atherogenesis.14 Consistently, infusion of activated wild-type, but not P-selectin–deficient platelets, into Apoe−/− mice increases atherosclerosis.6 In addition to modulating migration and recruitment of leukocytes, platelet/leukocyte interactions could potentially impact atherosclerosis and atherothrombosis by modulating other activities of leukocytes. Neutrophil extracellular traps (NETs) generated during NETosis promote venous and arterial thrombosis in animal models15,16 and recent evidence suggests a role of NETs in atherogenesis and atherothrombotic disorders as well.17-21 Interestingly, activated platelets interact with neutrophils and promote NETosis, which appears to require P-selectin/P-selectin glycoprotein ligand 1 interactions.22 Thus, activated platelets could potentially promote atherosclerosis and thrombogenesis by facilitating NET generation. Other roles of platelets in atherosclerosis have been reviewed and discussed elsewhere.2,23
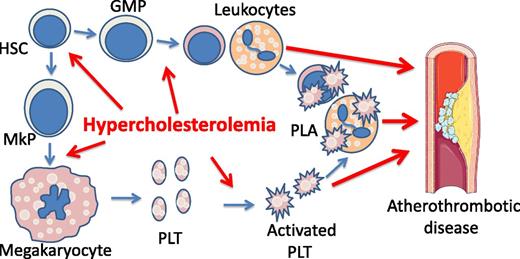
Hypercholesterolemia is a risk factor for atherothrombotic disease by promoting platelet production and activation. Hypercholesterolemia promotes megakaryopoiesis, platelet (PLT) biogenesis, and myelopoiesis, leading to leukocytosis. Hypercholesterolemia also increases platelet activation, likely by elevating platelet production and by direct impact on platelets. Activated platelets form PLAs, which are further increased in leukocytosis. PLA is an independent risk factor for atherothrombotic disease. GMP, guanosine monophosphate; HSC, hematopoietic stem cell; MkP, megakaryocyte progenitor cell.
Hypercholesterolemia is a risk factor for atherothrombotic disease by promoting platelet production and activation. Hypercholesterolemia promotes megakaryopoiesis, platelet (PLT) biogenesis, and myelopoiesis, leading to leukocytosis. Hypercholesterolemia also increases platelet activation, likely by elevating platelet production and by direct impact on platelets. Activated platelets form PLAs, which are further increased in leukocytosis. PLA is an independent risk factor for atherothrombotic disease. GMP, guanosine monophosphate; HSC, hematopoietic stem cell; MkP, megakaryocyte progenitor cell.
Cholesterol and platelet activation
Hyperlipidemia as exemplified by familial hypercholesterolemia is associated with increased platelet activation and an underlying procoagulant state.24-26 Hyperlipidemia primes platelets and increases platelet activation in response to various agonists.24,27 Plasma cholesterol levels appear to have a critical role in modulating platelet activity because hypercholesterolemia increases platelet activation more potently than hypertriglyceridemia.24,27 Hyperlipidemia increases platelet activation likely via multiple mechanisms.28,29 Oxidized low-density lipoprotein or oxidized phospholipids, which are increased in hyperlipidemia,28,30 serve as ligands of platelet CD36 and activate platelets.28,31 Oxidized lipids also promote formation of procoagulant tissue factor microparticles derived from monocytes.26 In vitro cholesterol loading also increases human platelet activation.32 High-density lipoprotein (HDL) is a cholesterol acceptor and promotes cholesterol efflux.33 HDL has been shown to mediate various antithrombotic effects, although some of the effects are via the impact on the vasculature, such as reducing endothelial cell surface expression of adhesion molecules.34 Infusions of a reconstituted HDL (rHDL) preparation reduced ex vivo platelet activation in diabetic subjects, likely by promoting cholesterol efflux from platelets.35 These findings in humans are consistent with observations in animal studies. A striking example is the markedly increased platelet activation and thrombosis in Scarbi−/− mice.29,36 Scarbi−/− mice have an unusually high plasma ratio of unesterified cholesterol to total cholesterol, reflecting impaired delivery of cholesterol by HDL from plasma and peripheral tissues back to the liver via hepatic scavenger receptor class B type I (SR-BI).37 Although the major function of hepatic SR-BI is to mediate selective uptake of cholesterol and cholesteryl ester from HDL, SR-BI-mediated cellular unesterified cholesterol efflux to HDL has been reported.38,39 The net unesterified cholesterol influx or efflux facilitated by SR-BI likely depends on the type of cells and the relative cholesterol/phospholipid ratio in the cell membrane vs that in HDL.40,41 SR-BI is expressed in platelets, whereas platelet cholesterol overload due to markedly increased unesterified cholesterol content in plasma lipoproteins, but not intrinsic SR-BI deficiency in platelets or other hematopoietic cells, is responsible for the heightened platelet activation in Scarb1−/− mice.29 As a consequence, Scarb1−/− mice developed spontaneous occlusive arterial thrombosis and premature death when introduced into hypercholesterolemic apoE−/− or Ldlr−/− background.42,43 Despite this accumulating evidence indicating a direct effect of cholesterol enrichment on platelet activation, the underlying mechanisms have not been elucidated. Cholesterol accumulation in plasma membranes disturbs membrane structures, particularly the cholesterol-rich specialized microdomains of lipid rafts.44 Enhanced signaling of cell surface receptors located in lipid rafts has been reported in various hematopoietic effector cells in response to membrane cholesterol accumulation.45,46 Thus, plasma membrane cholesterol accumulation in platelets could potentially alter the membrane structure and affect signaling via surface receptors. One study suggests that rHDL infusions suppress platelet activation by reducing lipid raft assembly.35 SR-BI is a receptor for HDL, and there is evidence that HDL suppresses thrombin-induced platelet aggregation by binding to SR-BI and generating inhibitory signals.47 In contrast, rHDL can suppress platelet activation via a mechanism independent of SR-BI,35 likely due to the fact that the cholesterol-free, phospholipid-rich rHDL can promote passive cholesterol efflux independent of transporters such as adenosine triphosphate–binding cassette transporter (ABC)A1, ABCG1, or SR-BI.
Cholesterol and platelet biogenesis
Platelets are derived from megakaryocytes, and the latter from MkPs in the bone marrow and spleen.48 Thrombopoietin (TPO) and its cognate receptor MPL proto-oncogene (c-MPL) act as a key growth-factor signaling pathway in megakaryopoiesis and platelet biogenesis.49 Genetic deficiency of TPO or c-MPL causes marked thrombocytopenia,49 whereas increased signaling in this pathway results in elevated platelet production and thrombocytosis.50 Plasma lipid levels have long been linked to platelet biogenesis and/or turnover. An analysis of 2 independent studies involving ∼10 000 participants indicates a positive correlation of non-HDL cholesterol levels with platelet counts,51 consistent with some other studies.52,53 Hyperlipidemia also is associated with shortened platelet survival and increased turnover, particularly in the setting of overt atherosclerosis.54-56 Together, these findings suggest the promotion of platelet production by hypercholesterolemia (Figure 1). TPO and c-MPL function to maintain platelet homeostasis, with TPO/c-MPL signaling in hematopoietic stem cells, MkPs, and megakaryocytes regulating megakaryocyte and platelet production, and megakaryocyte and platelet c-MPL acting as a sink for plasma TPO and mediating its internalization and turnover.49 Recent studies indicate an additional mechanism regulating platelet production in which aged desialylated platelets bind the hepatic Ashwell-Morell receptor and thereby stimulate hepatocyte TPO production.57,58 Increased platelet turnover is often associated with increased platelet biogenesis via elevated TPO. The newly generated platelets are larger, more dense, and RNA rich, and these so-called reticulated platelets are generally more active than the more mature platelets.59 Hypercholesterolemia is positively associated with the mean volume of platelets and ploidy of megakaryocytes in humans,60 suggesting the possibility that hypercholesterolemia primes platelets and increases platelet activity by promoting platelet production. Increased platelet activation is expected to increase the risk of coronary heart disease. Consistently, there is strong evidence that mean platelet volume and counts of reticulated platelets are positively associated with acute coronary syndrome,59 and 1 study shows that baseline platelet count is an independent risk factor for acute coronary syndrome.61 These findings in humans have been recapitulated in animal studies. Unesterified cholesterol accumulation in platelets of Scarbi−/− mice led to increased platelet turnover and clearance from circulation,62 which resulted in increased platelet biogenesis and produced juvenile platelets with greatly increased volume,29,62 likely as a consequence of the feedback regulation. Thus, the highly activated platelets in the circulation in Scarbi−/− mice29 could be the result of the combined effect of increased platelet biogenesis and the direct impact of platelet cholesterol enrichment on platelet activation. Moreover, dietary hypercholesterolemia led to thrombocytosis and leukocytosis as a result of bone marrow hematopoietic progenitor cell mobilization and altered interactions of megakaryocytes with endothelial cells due to disturbed CXCL12 and CXC4 signaling.63 However, the diet used in the study contained a very high cholesterol content, as well as cholate, and was proinflammatory; thus, effects may not be directly attributable to a direct impact of cholesterol in bone marrow progenitor populations. A recent study indicates that damage-associated molecular patterns promote bone marrow hematopoietic progenitor cell mobilization and extramedullary hematopoiesis.45 Nevertheless, another study assessed the impact of hypercholesterolemia induced by a high-fat, high-cholesterol diet in Ldlr−/− mice relative to the chow-fed Ldlr−/− mice on hematopoietic cells and showed an expansion of the pool of bone marrow hematopoietic stem and progenitor cells (HSPCs), in association with increased myelopoiesis.64 This study in younger mice examined platelet counts and found no change. However, we have detected increased platelet counts in ∼12-month-old chow-fed Ldlr−/− mice (∼12% increase; N.W., unpublished observation). Despite these observations, how hypercholesterolemia modulates platelet biogenesis or turnover at the molecular level remains enigmatic.
Mechanisms of cholesterol homeostasis in MkPs
Cholesterol homeostasis in hematopoietic cells is maintained in part by mechanisms involving ABC transporters and apolipoproteins (such as apolipoprotein E) that promote cellular cholesterol efflux.65 We showed increased myelopoiesis in ABCA1/ABCG1-deficient or apolipoprotein E–deficient mice due to increased cellular cholesterol accumulation in HSPCs as a result of defective cholesterol efflux.46,66 At the molecular level, increased myelopoiesis was attributed to the increased cell surface levels and signaling of common β subunit of interleukin-3 and granulocyte macrophage–colony-stimulating factor receptors.46 Increased myelopoiesis resulted in leukocytosis and accelerated atherosclerosis in these models.46,66 In subsequent studies in hypercholesterolemic Ldlr−/− mice, we found that deficiency of hematopoietic ABCG4, a transporter highly homologous to ABCG1 and actively promoting cholesterol efflux to HDL,67 increased atherosclerosis in association with increased platelet counts but without any change of plasma TPO levels.68 Selective thrombocytosis in bone marrow ABCG4 deficiency in combination with restricted Abcg4 expression in MkPs but with no or low expression in platelets suggested selectively increased megakaryopoiesis and platelet production. Indeed, MkPs and megakaryocytes, but not HSPCs or progenitors of other hematopoietic lineages, were increased in Abcg4−/− mice. As a result, hematopoietic ABCG4 deficiency led to defective cholesterol efflux to HDL and increased free cholesterol accumulation in MkPs (including plasma membranes) in association with increased c-MPL levels on the surface of Abcg4−/− MkPs, increased cell proliferation in response to TPO, and increased megakaryopoiesis and a more pronounced increase in platelet counts in response to TPO injection. The increased cell surface c-MPL levels in Abcg4−/− MkPs were because of blunting of the negative feedback regulation of c-MPL in response to TPO69 and involved a defective activation of Lyn kinase and casitas B-lineage lymphoma (c-CBL) E3 ligase. Lyn kinase, a palmitoylated membrane protein, seems to act as a membrane cholesterol sensor. Increased membrane cholesterol in Abcg4−/− MkPs may increase Lyn association with the membrane and decrease its tyrosine kinase activity in response to TPO,70 causing defective phosphorylation of c-CBL. This disrupts the negative feedback regulation of c-MPL and leads to increased TPO/c-MPL signaling and platelet production (Figure 2). In addition to increased atherosclerosis, Abcg4−/− mice showed accelerated arterial thrombosis in association with increased reticulated platelets, platelet/leukocyte complexes, and platelet-derived microparticles, all with proven proatherosclerotic and prothrombotic properties. These studies link increased platelet production, initiated from aberrant cholesterol metabolism in its lineage progenitor cells, to accelerated atherosclerosis and arterial thrombosis.
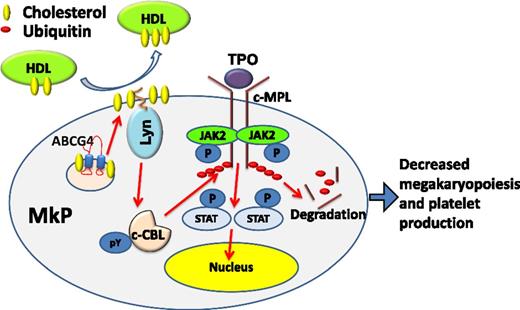
Working model. HDL-mediated cholesterol efflux from MkPs via ABCG4 increases activity of the palmitoylated Lyn kinase, c-CBL phosphorylation and activation, and c-MPL degradation, limiting MkP proliferation and platelet production. JAK2, Janus kinase 2; P, phosphate; pY, phosphotyrosine; STAT, signal transducer and activator of transcription.
Working model. HDL-mediated cholesterol efflux from MkPs via ABCG4 increases activity of the palmitoylated Lyn kinase, c-CBL phosphorylation and activation, and c-MPL degradation, limiting MkP proliferation and platelet production. JAK2, Janus kinase 2; P, phosphate; pY, phosphotyrosine; STAT, signal transducer and activator of transcription.
Infusion of rHDL reduced MkP proliferation and platelet counts in wild-type mice, but not in Abcg4−/− mice. The therapeutic potential of rHDL infusions in the control of platelet overproduction was exemplified by the finding that in a mouse model of essential thrombocythemia induced by bone marrow cell expression of a mutant form of c-MPL found in human subjects with essential thrombocythemia,71 rHDL reduced the platelet count in mice receiving Abcg4+/+, but not Abcg4−/−, bone marrow cells.68
Like ABCG4, ABCB6 is highly expressed in MkPs, and hematopoietic ABCB6 deficiency increases atherogenesis in hypercholesterolemic Ldlr−/− mice in association with selectively increased MkPs, megakaryocytes, total and reticulated platelet counts, and platelet activity.72 Unlike ABCG4, ABCB6 is reported to have transporter activity for porphyrin, but not for cholesterol.73 The detailed molecular mechanism linking ABCB6 deficiency to increased MkP proliferation and platelet production is unknown, but increased oxidative stress in Abcb6−/− MkPs has been suggested to contribute to these phenotypes.
In summary, platelets have critical roles in atherosclerosis and atherothrombosis. The accumulation of cholesterol in platelets or their progenitors, reflecting hypercholesterolemia or defective cholesterol efflux pathways, markedly increases platelet biogenesis, turnover, and activity, potentially contributing to atherogenesis and atherothrombosis. Although the mechanistic understanding of these processes is at an early stage, gaining further insight into the regulation of platelet production and activation by cholesterol and other lipids could open a new window on treatments for atherothrombosis.
Acknowledgments
The authors thank Andrew Murphy, Laurent Yvan-Charvet, and Prabhakara R. Nagareddy for discussion of this manuscript.
This work is supported by National Institutes of Health National Heart, Lung, and Blood Institute grants HL118567 (N.W.) and HL107653 (A.R.T.).
Authorship
Contribution: N.W. and A.R.T. wrote the manuscript.
Conflict-of-interest-disclosure: The authors declare no competing financial interests.
Correspondence: Nan Wang, Division of Molecular Medicine, Department of Medicine, Columbia University Medical Center, 630 W. 168th St, New York, NY 10032; e-mail: nw30@cumc.columbia.edu.