Key Points
Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL.
Updated results on safety and efficacy of the CLL8 trial.
Abstract
Despite promising results with targeted drugs, chemoimmunotherapy with fludarabine, cyclophosphamide (FC), and rituximab (R) remains the standard therapy for fit patients with untreated chronic lymphocytic leukemia (CLL). Herein, we present the long-term follow-up of the randomized CLL8 trial reporting safety and efficacy of FC and FCR treatment of 817 treatment-naïve patients with CLL. The primary end point was progression-free survival (PFS). With a median follow-up of 5.9 years, median PFS were 56.8 and 32.9 months for the FCR and FC group (hazard ratio [HR], 0.59; 95% confidence interval [CI], 0.50-0.69, P < .001). Median overall survival (OS) was not reached for the FCR group and was 86.0 months for the FC group (HR, 0.68; 95% CI, 0.54-0.89, P = .001). In patients with mutated IGHV (IGHV MUT), FCR improved PFS and OS compared with FC (PFS: HR, 0.47; 95% CI, 0.33-0.68, P < .001; OS: HR, 0.62; 95% CI, 0.34-1.11, P = .1). This improvement remained applicable for all cytogenetic subgroups other than del(17p). Long-term safety analyses showed that FCR had a higher rate of prolonged neutropenia during the first year after treatment (16.6% vs 8.8%; P = .007). Secondary malignancies including Richter’s transformation occurred in 13.1% in the FCR group and in 17.4% in the FC group (P = .1). First-line chemoimmunotherapy with FCR induces long-term remissions and highly relevant improvement in OS in specific genetic subgroups of fit patients with CLL, in particular those with IGHV MUT. This trial was registered at www.clinicaltrials.gov as #NCT00281918.
Introduction
Currently, there remains a general consensus that no curative treatment of chronic lymphocytic leukemia (CLL) exists with the exception of allogeneic stem cell transplantation.1 Chemoimmunotherapy achieves disease control and survival prolongation and is therefore the current standard treatment in patients with previously untreated CLL.2-5 In the last 5 years, 2 new drugs, ibrutinib and idelalisib, targeting B-cell receptor (BCR) signaling, have been studied and approved. They are less toxic in the short term than chemotherapeutic agents and yielded promising responses in patients with relapsed/refractory CLL including those with a TP53 aberration.6-11 Furthermore, the selective BCL-2 inhibitor venetoclax is currently being investigated in ongoing clinical trials.12 Given these results, it is likely that the place of first-line chemoimmunotherapy may be challenged in the future. Importantly, the experience with these new drugs remains limited, and the current treatment model requires continuous indefinite treatment. Moreover, there is a lack of prospective, controlled trials with these agents in the first-line setting.13 Additionally, although the outcome following chemoimmunotherapy may be highly variable, important subgroups of patients show an excellent outcome, corroborating its use as standard first-line therapy. On the other hand, secondary malignancies are one of the most concerning unwanted effects following chemoimmunotherapy, and the true long-term incidence needs to be better defined.
We have previously shown that the addition of the anti-CD20 monoclonal antibody rituximab (R) to fludarabine and cyclophosphamide (FC), improved both the progression-free survival (PFS) and the overall survival (OS) of physically fit patients with previously untreated, symptomatic CLL.2 On the basis of these findings, FCR has become the standard therapy for physically fit patients with previously untreated CLL. In this report, we present the results of an extended observation time with a median follow-up of 5.9 years of the randomized phase 3 CLL8 trial of the German CLL Study Group (GCLLSG), with particular emphasis on long-term follow-up for survival and adverse events.
Methods
Study design
The study design has been reported previously.2 In short, the CLL8 trial was a prospective, randomized, open-label, phase 3 study conducted at 190 centers across 11 countries. The Institutional Review Board and/or ethics committee of each institution approved the study protocol. Each patient provided written informed consent prior to enrollment. Study treatment consisted of 6 courses (28 days/course) of intravenous F (25 mg/m2 per day) and C (250 mg/m2 per day) for the first 3 days of each treatment course, with or without the addition of rituximab. Rituximab (Mabthera/Rituxan; F. Hoffmann-La Roche Ltd.) was administered at a dose of 375 mg/m2 on day 0 of course 1 and 500 mg/m2 on day 1 of courses 2 to 6. Neither antiviral prophylaxis nor prophylactic use of granulocyte-colony stimulating factor was recommended in this study (supplemental Information available on the Blood Web site). Pharmacologic prophylaxis of Pneumocystis carinii (Pneumocystis jiroveci) pneumonia was recommended in case of prolonged severe leukocytopenia (>7 days). An initial response assessment after completion of therapy was performed 1 month ± 7 days after the start of the last course of therapy. The results obtained were confirmed at the final assessment, performed ≥2 months later. Subsequently, patients completed follow-up examinations every 3 months for the ensuing years 1 to 3, every 6 months for years 4 and 5, and annually up to year 8.
Role of the funding source
This trial was planned and initiated in 2003 as an investigator-initiated trial by the GCLLSG. From 2004, Hoffmann-La Roche Ltd. assumed sponsorship for this trial.
Patients
Between 2003 and 2006, the study enrolled treatment-naïve patients with immunophenotypically confirmed CLL with Binet stage C,14 or Binet stages A and B with confirmed active disease.15 Additional inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, a low comorbidity as defined by a cumulative illness rating scale (CIRS)16 of up to 6, and a creatinine clearance ≥70 mL/min (see supplemental Data). Major exclusion criteria were the absence of active disease in patients with Binet stage A or B and the presence of clinically apparent autoimmune cytopenia or active second malignancy.
Study procedures and end points
Comprehensive assessments, including confirmation of diagnosis by flow cytometry, tumor assessments, and CIRS and ECOG performance status, were completed at baseline. The central reference laboratory (Ulm, Germany) analyzed genomic aberrations by fluorescent in situ hybridization (FISH)17 and IGHV mutational status18,19 by DNA sequencing. Response to treatment and disease progression was classified according to the National Cancer Institute working group criteria.15 Responses and disease progression were assessed by the study investigators and verified by a central, independent medical review. The primary end point of this study was PFS. Secondary end points of the study included OS, safety including rates of treatment-related adverse effects, and survival times in biological subgroups. This report will present the final primary efficacy analysis (PFS), long-term safety results including the incidence of secondary malignancies, and results of major secondary end points such as OS.
Statistical analysis
Time to event end points were analyzed using the Kaplan-Meier method, and comparisons of survival curves were performed with the log-rank test. Hazard ratios (HRs) including 95% confidence intervals (CI) were calculated using proportional-hazards Cox regressions. OS was calculated from the date of randomization to death from any cause and PFS from randomization to disease progression or death. Time to second primary malignancy (SPM) was defined as the time between start of study treatment to date of first diagnosis of SPM. Patients who died without a SPM were censored at date of death and patients without documented event were censored at date of last information. The median observation time was calculated for patients alive from randomization. The standardized mortality ratio (SMR) was calculated by comparing the mortality of the male and female patients to the corresponding German male and female population. Expected mortality was estimated considering calendar year- (1998-2013), sex-, and age group-specific rates from the mortality table 2009/2011 of the German Federal Office of Statistics. Person-years at risk were calculated for each patient from randomization until the end of observation, which was defined as date of death or last date of last information. Standardized incidence ratios were similarly calculated based on the incidence table 2012 of the German Society of Epidemiological Cancer Registry. In terms of prognostic factors, univariate and multivariate proportional-hazards Cox regression analyses were applied to PFS and OS.
To determine the frequency of prolonged grade 3 or 4 neutropenia, absolute neutrophils counts collected after end of treatment were analyzed. Values subsequent to a new treatment of CLL were excluded. Neutrophil counts between 0.5 and 1.0 × 109/L were classified as grade 3 and those <0.5 × 109/L were classified as grade 4.
Patients were considered as having prolonged grade 3 to 4 neutropenia during the first year after the end of treatment if ≥1 grade 3 or grade 4 neutropenia according to absolute neutrophils occurred between 2 and 12 months after the end of treatment. Patient groups were compared by χ2, Fisher’s exact, or nonparametric rank-sum tests as appropriate. All statistical tests were 2 sided, and statistical significance was defined as P < .05. Adjustments for multiple comparisons were not applied. The analysis was performed with SPSS V21.0 and SAS 9.2. The analyses are based on a data cutoff in 2012.
Results
Patient demographics and baseline characteristics
The study included 817 patients randomly assigned to receive 6 courses of either FC or FCR. Results are now reported after a median observation time of 5.9 years (compared with 3.1 years in the first publication). Patients had a median age of 61 years, 31% had Binet stage C disease, 63% had an unmutated IGHV status (UNM), and 8.2% had a del(17p) (Table 1). Treatment arms were well balanced with regard to age, sex, disease stage, physical fitness, creatinine clearance, serum β2-microglobulin levels, genomic aberrations, and IGHV mutational status (Table 1). Centrally assessed genomic profiling data (FISH, IGHV mutational status, and gene mutations) were available for 635 (78%) patients (Table 1). This cohort was representative of the full trial population with respect to demographics and other baseline prognostic factors.
Patient demographics and baseline characteristics
| Characteristic . | FC . | FCR . | P value . |
|---|---|---|---|
| All patients (ITT), N | 409 | 408 | |
| Age | N = 409 | N = 408 | |
| Median, years | 61 | 61 | |
| Range, years | 36-81 | 30-80 | |
| ≥65 years, no. (%) | 119 (29) | 126 (31) | |
| ≥75 years, no. (%) | 37 (9) | 44 (11) | |
| Sex, no. (%) | N = 409 | N = 408 | |
| Male | 304 (74) | 303 (74) | |
| Binet stage, no. (%) | N = 407 | N = 407 | |
| A | 22 (5) | 18 (4) | |
| B | 259 (64) | 263 (64) | |
| C | 126 (31) | 126 (31) | |
| Presence of B symptoms, no. (%) | N = 406 | N = 407 | |
| Yes | 197 (49) | 167 (41) | |
| Cumulative illness rating scale | N = 409 | N = 408 | |
| Median | 1 | 1 | |
| Range | 0-8 | 0-7 | |
| ECOG performance status, no. (%) | N = 390 | N = 395 | |
| 0 | 226 (58) | 221 (56) | |
| Cytogenetic abnormalities, no. (%) | N = 305 | N = 311 | |
| 17p deletion | 29 (10) | 22 (7) | |
| 11q deletion | 62 (20) | 80 (26) | |
| Trisomy 12 | 37 (12) | 24 (8) | |
| Normal | 58 (19) | 80 (26) | |
| 13q deletion | 119 (39) | 105 (34) | |
| IGHV mutational status, no. (%) | N = 312 | N = 310 | |
| UNM | 195 (63) | 197 (64) | |
| MUT | 117 (37) | 113 (37) | |
| NOTCH1 mutation, no. (%) | N = 312 | N = 310 | |
| Mutated | 32 (10) | 30 (10) | |
| Wild type | 280 (90) | 280 (90) | |
| SF3B1 mutation, no. (%) | N = 312 | N = 310 | |
| Mutated | 59 (19) | 55 (18) | |
| Wild type | 253 (91) | 254 (82) | |
| Serum thymidine kinase level, no. (%) | N = 288 | N = 303 | |
| ≥10.0 U/L | 225 (78) | 222 (73) | |
| Serum β2- microglobulin level, no. (%) | N = 288 | N = 303 | |
| ≥3.5 mg/L | 95 (33) | 102 (34) | |
| Response to treatment, no. (%) | |||
| Complete response | 88 (22) | 180 (44) | <.001 |
| Overall response | 328 (80) | 369 (90) | <.001 |
| Missing response | 38 (9) | 20 (5) |
| Characteristic . | FC . | FCR . | P value . |
|---|---|---|---|
| All patients (ITT), N | 409 | 408 | |
| Age | N = 409 | N = 408 | |
| Median, years | 61 | 61 | |
| Range, years | 36-81 | 30-80 | |
| ≥65 years, no. (%) | 119 (29) | 126 (31) | |
| ≥75 years, no. (%) | 37 (9) | 44 (11) | |
| Sex, no. (%) | N = 409 | N = 408 | |
| Male | 304 (74) | 303 (74) | |
| Binet stage, no. (%) | N = 407 | N = 407 | |
| A | 22 (5) | 18 (4) | |
| B | 259 (64) | 263 (64) | |
| C | 126 (31) | 126 (31) | |
| Presence of B symptoms, no. (%) | N = 406 | N = 407 | |
| Yes | 197 (49) | 167 (41) | |
| Cumulative illness rating scale | N = 409 | N = 408 | |
| Median | 1 | 1 | |
| Range | 0-8 | 0-7 | |
| ECOG performance status, no. (%) | N = 390 | N = 395 | |
| 0 | 226 (58) | 221 (56) | |
| Cytogenetic abnormalities, no. (%) | N = 305 | N = 311 | |
| 17p deletion | 29 (10) | 22 (7) | |
| 11q deletion | 62 (20) | 80 (26) | |
| Trisomy 12 | 37 (12) | 24 (8) | |
| Normal | 58 (19) | 80 (26) | |
| 13q deletion | 119 (39) | 105 (34) | |
| IGHV mutational status, no. (%) | N = 312 | N = 310 | |
| UNM | 195 (63) | 197 (64) | |
| MUT | 117 (37) | 113 (37) | |
| NOTCH1 mutation, no. (%) | N = 312 | N = 310 | |
| Mutated | 32 (10) | 30 (10) | |
| Wild type | 280 (90) | 280 (90) | |
| SF3B1 mutation, no. (%) | N = 312 | N = 310 | |
| Mutated | 59 (19) | 55 (18) | |
| Wild type | 253 (91) | 254 (82) | |
| Serum thymidine kinase level, no. (%) | N = 288 | N = 303 | |
| ≥10.0 U/L | 225 (78) | 222 (73) | |
| Serum β2- microglobulin level, no. (%) | N = 288 | N = 303 | |
| ≥3.5 mg/L | 95 (33) | 102 (34) | |
| Response to treatment, no. (%) | |||
| Complete response | 88 (22) | 180 (44) | <.001 |
| Overall response | 328 (80) | 369 (90) | <.001 |
| Missing response | 38 (9) | 20 (5) |
Treatment efficacy
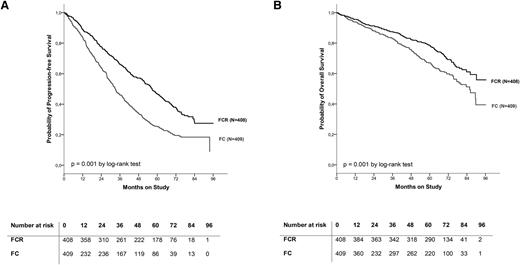
Updated analyses of time to event end points confirmed the superiority of chemoimmunotherapy. Median PFS was longer in the FCR group (56.8 months) than in the FC group (32.9 months; HR, 0.59; 95% CI, 0.50-0.69; P < .001; Figure 1). Patients with Binet stages A, B, and C disease each showed a similar median PFS of 34.1, 32.9, and 33.1 months, respectively, when treated with FC (supplemental Data). Treatment with FCR improved the median PFS to 58.2 months in Binet stage B (HR, 0.55; 95% CI, 0.45-0.68; P < .001) and to 42.5 months in Binet stage C (HR, 0.71; 95% CI, 0.53-0.95; P = .022). Median PFS was not reached in patients at Binet stage A treated with FCR. FCR therapy also resulted in a significant benefit with regard to OS. The median OS was 86.0 months for patients treated with FC, whereas median OS was not reached for patients treated with FCR (HR, 0.68; 95% CI, 0.54-0.89; P = .001; Figure 1) including more deaths after FC (154 of 409, 37.7%) than after FCR treatment (125 of 408, 30.6%). The most common causes of death were infections (sepsis and pulmonary infection being the most frequent events in both arms; FC 60 of 154, median time of 39 months to onset after last dose of study treatment; FCR 53 of 125, median time of 46 months to onset after last dose of study treatment) followed by progressive disease (FC 36 of 154, FCR 31 of 125) and secondary malignancies (solid tumor and lymphoma being the most frequent fatal events in the FC group and lymphoma being the most frequent event in the FCR group; FC 34 of 154, median time of 33 months to onset after last dose of study treatment; FCR 22 of 125, median time of 27 months to onset after last dose of study treatment). Other causes of death included myocardial infarction (FC 10 of 154, FCR 7 of 125) and renal and cerebrovascular events (FC 2 of 154, FCR 0 of 125). SMR was 5.4, indicating an increased risk of death in the study population compared with an age- and sex-matched general German population. Moreover, the SMR was higher in the FC than in the FCR group (FCR: SMR, 4.5; 95% CI, 3.7-5.3; FC: SMR, 6.5; 95% CI, 5.5-7.6).
PFS and OS in both treatment arms. (A) PFS in both treatment arms (FCR vs FC, all patients evaluable for PFS, N = 817); (B) OS in both treatment arms (FCR vs FC, all patients evaluable for OS: N = 817).
PFS and OS in both treatment arms. (A) PFS in both treatment arms (FCR vs FC, all patients evaluable for PFS, N = 817); (B) OS in both treatment arms (FCR vs FC, all patients evaluable for OS: N = 817).
Prognostic indicators of time to progression and survival
The benefit of FCR was observed especially in young individuals assessed by a statistically significant longer overall survival (FCR age <65 years: median OS not reached; FCR age ≥65 years: median OS, 80.8 months; HR, 0.62; 95% CI, 0.43-0.88; P = .009). This difference was not observed for PFS (FCR age <65 years: median PFS, 58.3 months; FCR age ≥65 years: median PFS, 55.4 months; HR, 0.89; 95% CI, 0.69-1.12; P = .41). No survival or PFS differences were observed for treatment with FC (FC age <65 years: median PFS, 33.0 months; FC age ≥65 years: median PFS, 32.9 months; HR, 1.02; 95% CI, 0.79-1.31; P = .896; FC age <65 years: median OS, 89.5 months; FC age ≥65 years: median OS, 78.9 months; HR, 0.79; 95% CI, 0.56-1.10; P = .166). FCR therapy resulted in significantly higher PFS and OS in most genetic subgroups, including del(17p), del(11q), del(13q), and trisomy 12 (data not shown). The del(17p) subgroup showed the shortest median PFS but still had treatment benefit from FCR (FCR, 11.2 months; FC, 9.1 months; HR, 0.49; 95% CI, 0.25-0.93; P = .03). Median OS for del(17p) patients treated with FCR was 33.1 and 23.0 months for those treated with FC (HR, 0.66; 95% CI, 0.33-1.31; P = .2). Subgroup analysis data on 5-year PFS and OS are shown in Table 2. For patients with normal karyotype, no statistically significant difference between FC and FCR for PFS and OS was observed (median PFS: FCR, 50.4 months; FC, 35.8 months; HR, 0.89; 95% CI, 0.20-0.81; P = .364; median OS: FCR, 11.2 months; FC, 9.1 months; HR, 1.31; 95% CI, 0.73-2.35; P = .37).
PFS and OS in prognostic subgroups
| Characteristics . | FCR 5-year rate, % . | FC 5-year rate, % . | HR (95% CI) . | P value . |
|---|---|---|---|---|
| PFS | ||||
| All patients (N = 817) | 46.8 | 25.5 | 0.59 (0.50-0.69) | <.001 |
| Age | ||||
| <65 years (N = 572) | 48.3 | 25.2 | 0.57 (0.47-0.70) | <.001 |
| ≥65 years (N = 245) | 43.2 | 26.1 | 0.63 (0.47-0.85) | .003 |
| Binet stage | ||||
| A (N = 40) | 60.2 | 28.9 | 0.44 (0.18-1.12) | .084 |
| B (N = 522) | 47.7 | 25.4 | 0.55 (0.46-0.68) | <.001 |
| C (N = 252) | 43.0 | 25.3 | 0.71 (0.53-0.95) | .023 |
| Sex | ||||
| Female (N = 210) | 58.8 | 33.1 | 0.58 (0.40-0.83) | .003 |
| Male (N = 607) | 42.6 | 23.0 | 0.59 (0.49-0.72) | <.001 |
| Cytogenetic abnormalities | ||||
| 17p deletion (N = 51) | 15.3 | 0.0 | 0.47 (0.25-0.90) | .023 |
| 11q deletion (N = 142) | 31.4 | 11.4 | 0.47 (0.32-0.68) | <.001 |
| Trisomy 12 (N = 61) | 61.6 | 23.7 | 0.41 (0.20-0.81) | .01 |
| Normal (N = 138) | 42.8 | 37.6 | 0.83 (0.54-1.26) | .365 |
| 13q deletion (N = 224) | 63.3 | 31.0 | 0.44 (0.31-0.62) | <.001 |
| IGHV mutational status | ||||
| UNM (N = 392) | 33.1 | 19.4 | 0.65 (0.52-0.82) | <.001 |
| MUT (N = 230) | 66.6 | 36.2 | 0.47 (0.33-0.68) | <.001 |
| NOTCH1 mutation | ||||
| Wild type (N = 560) | 48.0 | 25.3 | 0.55 (0.45-0.68) | <.001 |
| Mutated (N = 62) | 26.7 | 25.8 | 1.01 (0.57-1.78) | .974 |
| SF3B1 mutation | ||||
| Wild-type (N = 507) | 49.1 | 27.8 | 0.60 (0.48-0.74) | <.001 |
| Mutated (N = 114) | 31.3 | 14.9 | 0.53 (0.35-0.80) | .003 |
| OS | ||||
| All patients (N = 817) | 78.7 | 66.9 | 0.68 (0.54-0.89) | .001 |
| Age | ||||
| <65 years (N = 572) | 80.9 | 69.2 | 0.63 (0.47-0.84) | .002 |
| ≥65 years (N = 245) | 73.9 | 61.6 | 0.81 (0.54-1.20) | .288 |
| Binet stage | ||||
| A (N = 40) | 94.4 | 66.0 | 0.11 (0.01-0.84) | .034 |
| B (N = 522) | 82.1 | 66.8 | 0.59 (0.44-0.80) | .001 |
| C (N = 252) | 69.0 | 67.3 | 1.02 (0.68-1.53) | .918 |
| Sex | ||||
| Female (N = 210) | 81.3 | 64.5 | 0.56 (0.34-0.93) | .003 |
| Male (N = 607) | 77.8 | 67.8 | 0.71 (0.55-0.93) | <.001 |
| Cytogenetic abnormalities | ||||
| 17p deletion (N = 51) | 36.0 | 18.2 | 0.64 (0.32-1.25) | .19 |
| 11q deletion (N = 142) | 85.8 | 55.1 | 0.35 (0.20-0.61) | <.001 |
| Trisomy 12 (N = 61) | 91.5 | 77.4 | 0.54 (0.19-1.55) | .251 |
| Normal (N = 138) | 74.0 | 81.2 | 1.31 (0.73-2.35) | .370 |
| 13q deletion (N = 224) | 87.1 | 73.1 | 0.49 (0.28-0.84) | .01 |
| Characteristics . | FCR 5-year rate, % . | FC 5-year rate, % . | HR (95% CI) . | P value . |
|---|---|---|---|---|
| PFS | ||||
| All patients (N = 817) | 46.8 | 25.5 | 0.59 (0.50-0.69) | <.001 |
| Age | ||||
| <65 years (N = 572) | 48.3 | 25.2 | 0.57 (0.47-0.70) | <.001 |
| ≥65 years (N = 245) | 43.2 | 26.1 | 0.63 (0.47-0.85) | .003 |
| Binet stage | ||||
| A (N = 40) | 60.2 | 28.9 | 0.44 (0.18-1.12) | .084 |
| B (N = 522) | 47.7 | 25.4 | 0.55 (0.46-0.68) | <.001 |
| C (N = 252) | 43.0 | 25.3 | 0.71 (0.53-0.95) | .023 |
| Sex | ||||
| Female (N = 210) | 58.8 | 33.1 | 0.58 (0.40-0.83) | .003 |
| Male (N = 607) | 42.6 | 23.0 | 0.59 (0.49-0.72) | <.001 |
| Cytogenetic abnormalities | ||||
| 17p deletion (N = 51) | 15.3 | 0.0 | 0.47 (0.25-0.90) | .023 |
| 11q deletion (N = 142) | 31.4 | 11.4 | 0.47 (0.32-0.68) | <.001 |
| Trisomy 12 (N = 61) | 61.6 | 23.7 | 0.41 (0.20-0.81) | .01 |
| Normal (N = 138) | 42.8 | 37.6 | 0.83 (0.54-1.26) | .365 |
| 13q deletion (N = 224) | 63.3 | 31.0 | 0.44 (0.31-0.62) | <.001 |
| IGHV mutational status | ||||
| UNM (N = 392) | 33.1 | 19.4 | 0.65 (0.52-0.82) | <.001 |
| MUT (N = 230) | 66.6 | 36.2 | 0.47 (0.33-0.68) | <.001 |
| NOTCH1 mutation | ||||
| Wild type (N = 560) | 48.0 | 25.3 | 0.55 (0.45-0.68) | <.001 |
| Mutated (N = 62) | 26.7 | 25.8 | 1.01 (0.57-1.78) | .974 |
| SF3B1 mutation | ||||
| Wild-type (N = 507) | 49.1 | 27.8 | 0.60 (0.48-0.74) | <.001 |
| Mutated (N = 114) | 31.3 | 14.9 | 0.53 (0.35-0.80) | .003 |
| OS | ||||
| All patients (N = 817) | 78.7 | 66.9 | 0.68 (0.54-0.89) | .001 |
| Age | ||||
| <65 years (N = 572) | 80.9 | 69.2 | 0.63 (0.47-0.84) | .002 |
| ≥65 years (N = 245) | 73.9 | 61.6 | 0.81 (0.54-1.20) | .288 |
| Binet stage | ||||
| A (N = 40) | 94.4 | 66.0 | 0.11 (0.01-0.84) | .034 |
| B (N = 522) | 82.1 | 66.8 | 0.59 (0.44-0.80) | .001 |
| C (N = 252) | 69.0 | 67.3 | 1.02 (0.68-1.53) | .918 |
| Sex | ||||
| Female (N = 210) | 81.3 | 64.5 | 0.56 (0.34-0.93) | .003 |
| Male (N = 607) | 77.8 | 67.8 | 0.71 (0.55-0.93) | <.001 |
| Cytogenetic abnormalities | ||||
| 17p deletion (N = 51) | 36.0 | 18.2 | 0.64 (0.32-1.25) | .19 |
| 11q deletion (N = 142) | 85.8 | 55.1 | 0.35 (0.20-0.61) | <.001 |
| Trisomy 12 (N = 61) | 91.5 | 77.4 | 0.54 (0.19-1.55) | .251 |
| Normal (N = 138) | 74.0 | 81.2 | 1.31 (0.73-2.35) | .370 |
| 13q deletion (N = 224) | 87.1 | 73.1 | 0.49 (0.28-0.84) | .01 |
This updated analysis showed that FC therapy, del(17p), IGHV UNM status, serum thymidine kinase (s-TK) ≥ 10 U/L, del(11q), mutated (MUT) TP53, and MUT SF3B1 were independently associated with shorter PFS in Cox regression analysis (Table 3). Similarly, FC therapy, TK ≥ 10 U/L, s-β2m ≥ 3.5 mg/L, del(17p), ECOG performance status > 0, age ≥65 years, IGHV UNM, and MUT TP53 status were independent adverse prognostic factors for OS (Table 3). The presence of MUT TP53, del(17p), and IGHV UNM showed the strongest prognostic impact on both PFS and OS as reflected by the HR.
Final multivariate analysis of the effects of various prognostic factors on PFS and OS
| Characteristic . | Adverse factor . | HR . | 95% CI . | P value . |
|---|---|---|---|---|
| PFS (N = 500; 348 [42.6%] events) | ||||
| Study treatment | FC | 1.976 | 1.59-2.45 | <.001 |
| Serum thymidine kinase level | ≥10 U/L | 1.362 | 1.10-1.77 | .020 |
| IGHV mutational status | UNM | 1.719 | 1.33-2.23 | <.001 |
| Cytogenetic subgroup | Del(11q) | 1.546 | 1.22-1.97 | <.001 |
| Cytogenetic subgroup | Del(17p) | 2.916 | 1.78-4.78 | <.001 |
| TP53 mutational status | Mutated | 2.123 | 1.40-3.22 | <.001 |
| SF3B1 mutational status | Mutated | 1.346 | 1.04-1.75 | .026 |
| OS (N = 500; 173 [21.2%] events) | ||||
| Study treatment | FC | 1.538 | 1.14-2.08 | .006 |
| Age | ≥65 y | 1.423 | 1.04-1.20 | .018 |
| ECOG | >0 | 1.622 | 1.20-2.21 | .002 |
| Serum β2- microglobulin level | ≥3.5 mg/L | 1.473 | 1.07-2.03 | .014 |
| Serum thymidine kinase level | ≥10 U/L | 1.864 | 1.20-2.90 | .003 |
| IGHV mutational status | UNM | 2.059 | 1.39-3.05 | <.001 |
| Cytogenetic subgroup | Del(17p) | 2.715 | 1.60-4.60 | <.001 |
| TP53 mutational status | Mutated | 3.014 | 1.89-4.80 | <.001 |
| Characteristic . | Adverse factor . | HR . | 95% CI . | P value . |
|---|---|---|---|---|
| PFS (N = 500; 348 [42.6%] events) | ||||
| Study treatment | FC | 1.976 | 1.59-2.45 | <.001 |
| Serum thymidine kinase level | ≥10 U/L | 1.362 | 1.10-1.77 | .020 |
| IGHV mutational status | UNM | 1.719 | 1.33-2.23 | <.001 |
| Cytogenetic subgroup | Del(11q) | 1.546 | 1.22-1.97 | <.001 |
| Cytogenetic subgroup | Del(17p) | 2.916 | 1.78-4.78 | <.001 |
| TP53 mutational status | Mutated | 2.123 | 1.40-3.22 | <.001 |
| SF3B1 mutational status | Mutated | 1.346 | 1.04-1.75 | .026 |
| OS (N = 500; 173 [21.2%] events) | ||||
| Study treatment | FC | 1.538 | 1.14-2.08 | .006 |
| Age | ≥65 y | 1.423 | 1.04-1.20 | .018 |
| ECOG | >0 | 1.622 | 1.20-2.21 | .002 |
| Serum β2- microglobulin level | ≥3.5 mg/L | 1.473 | 1.07-2.03 | .014 |
| Serum thymidine kinase level | ≥10 U/L | 1.864 | 1.20-2.90 | .003 |
| IGHV mutational status | UNM | 2.059 | 1.39-3.05 | <.001 |
| Cytogenetic subgroup | Del(17p) | 2.715 | 1.60-4.60 | <.001 |
| TP53 mutational status | Mutated | 3.014 | 1.89-4.80 | <.001 |
Variables included in the model applying backward selection (complete case analysis): type of therapy (FC/FCR), age, sex, disease stage, ECOG performance status, B symptoms, white blood cell count, s-TK, s-β2m, del(11q), trisomy 12, del(13q), del(17p), IGHV mutation status, TP53 mutation status [irrespective del(17p)], NOTCH1 mutation status, and SF3B1 mutation status (N = 507).
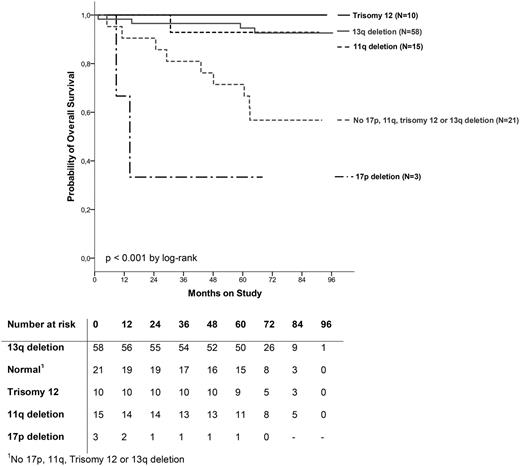
PFS in patients with IGHV MUT/UNM genes showed a significant difference in favor of the IGHV MUT subgroup. The IGHV MUT group treated with FCR had a significantly longer PFS than those treated with FC (median PFS FCR, not reached; FC, 41.9 months, HR, 0.47; 95% CI, 0.33-0.68; P < .001; Figure 2). The median OS for IGHV MUT and UNM CLL was not reached (HR, 0.62; 95% CI, 0.34-1.11; P = .1; Figure 2). The OS analysis showed that the OS rate of the IGHV MUT patients treated with FCR was 86.3% after 5 years compared with 79.8% treated with FC. The analysis of cytogenetic subgroups within IGHV MUT patients treated with FCR confirmed this good long-term outcome for most cytogenetic subgroups, except for patients with a del(17p) or patients without FISH abnormalities (Figure 3). Median OS for IGHV MUT CLL with a del(11q), a trisomy 12, del(13q), or a normal karyotype was not reached. Median OS was 14.3 months for IGHV MUT CLL patients with a del(17p).
PFS and OS in both treatment arms and IGHV MUT/UNM patients. (A) PFS in both treatment arms and IGHV MUT/UNM patients (FCR vs FC, all patients, N = 622); (B) OS in both treatment arms and IGHV MUT/UNM patients (FCR vs FC, all patients, N = 622).
PFS and OS in both treatment arms and IGHV MUT/UNM patients. (A) PFS in both treatment arms and IGHV MUT/UNM patients (FCR vs FC, all patients, N = 622); (B) OS in both treatment arms and IGHV MUT/UNM patients (FCR vs FC, all patients, N = 622).
Results of MRD assessments in peripheral blood assessed by 4-color flow cytometry at final restaging (3 months after start of last treatment course) were available in 37% (85 of 230) of IGHV MUT patients. Of these, 68% obtained a MRD negative remission (<10−4) with FCR treatment at final restaging compared with 38% with FC (P = .006).
Long-Term Safety
All patients who received ≥1 dose of any study drug were included in the safety analysis (N = 800; Table 4). FCR treatment was associated with a higher rate of prolonged grades 3 and 4 neutropenia within 1 year after the end of treatment (67 of FCR treated patients [16.8%] vs 34 FC patients [8.8%]; P = .007). At 12 months or more after treatment, rates of prolonged neutropenia were not different in the 2 treatment arms (FCR 16 [4.0%]; FC 14 [3.5%]; P = .75; Table 4).
Long-term safety including prolonged neutropenia and secondary malignancies
| Long-term safety . | Total . | FC . | FCR . | |||
|---|---|---|---|---|---|---|
| Cases N (%) . | Patients N (%) . | Cases N (%) . | Patients N (%) . | Cases N (%) . | Patients N (%) . | |
| Total patients (safety population), N | 800 | 396 | 404 | |||
| Total cases [N (%)] and patients [N (%)] with ≥1 SPM | 136 (100) | 122 (15) | 77 (57) | 69 (17) | 59 (43) | 53 (13) |
| Secondary malignancies | ||||||
| Richter’s transformation | 38 (28) | 38 (5) | 25 (33) | 25 (6) | 13 (22) | 13 (3) |
| Solid tumors | 55 (40) | 52 (7) | 29 (38) | 28 (7) | 26 (44) | 24 (6) |
| Lung | 18/55 (33) | 18 (2) | 13/29 (45) | 13 (3) | 5/26 (20) | 5 (1) |
| Prostate | 8/55 (15) | 8 (1) | 2/29 (7) | 2 (1) | 6/26 (23) | 6 (2) |
| Renal/bladder | 7/55 (13) | 6 (1) | 3/29 (10) | 3 (1) | 4/26 (15) | 3 (1) |
| Colorectal | 2/55 (4) | 2 (<1) | 0/29 (0) | 0 (0) | 2/26 (8) | 2 (<1) |
| Melanoma | 8/55 (15) | 8 (1) | 3/29 (10) | 3 (1) | 5/26 (20) | 5 (1) |
| Breast | 3/55 (6) | 3 (<1) | 1/29 (3) | 1 (<1) | 2/26 (8) | 2 (<1) |
| Pancreatic | 2/55 (4) | 2 (<1) | 1/29 (3) | 1 (<1) | 1/26 (4) | 1 (<1) |
| Ovarian/uterine/cervical | 1/55 (2) | 1 (<1) | 0/29 (0) | 0 (0) | 1/26 (4) | 1 (<1) |
| Liver/gall bladder | 1/55 (2) | 1 (<1) | 1/29 (3) | 1 (<1) | 0/26 (0) | 0 (0) |
| Thyroid | 2/55 (4) | 2 (<1) | 2/29 (7) | 2 (1) | 0/26 (0) | 0 (0) |
| Pharyngeal/laryngeal | 1/55 (2) | 1 (<1) | 1/29 (3) | 1 (<1) | 0/26 (0) | 0 (0) |
| Other | 2/55 (4) | 2 (<1) | 2/29 (7) | 2 (1) | 0/26 (0) | 0 (0) |
| Hematologic neoplasia | 24 (18) | 23 (3) | 11 (14) | 11 (3) | 13 (22) | 12 (3) |
| AML/MDS | 14/24 (58) | 13 (2) | 7/11 (64) | 7 (2) | 7/13 (54) | 6 (2) |
| Indolent B-non-Hodgkin lymphoma | 3/24 (13) | 3 (<1) | 1/11 (9) | 1 (<1) | 2/13 (16) | 2 (<1) |
| Aggressive B-non-Hodgkin lymphoma | 2/24 (8) | 2 (<1) | 1/11 (9) | 1 (<1) | 1/13 (8) | 1 (<1) |
| ALL | 1/24 (4) | 1 (<1) | 0/11 (0) | 0 (0) | 1/13 (8) | 1 (<1) |
| CML | 1/24 (4) | 1 (<1) | 0/11 (0) | 0 (0) | 1/13 (8) | 1 (<1) |
| Other | 3/24 (13) | 3 (<1) | 2/11 (18) | 2 (<1) | 1/13 (8) | 1 (<1) |
| Basalioma, squamous cell | 19 (14) | 17 (2) | 12 (16) | 11 (3) | 7 (12) | 6 (2) |
| Prolonged neutropenia | ||||||
| 2 months after end of treatment | 101 (13) | 34 (9) | 67 (17) | |||
| 12 months after end of treatment | 30 (4) | 14 (4) | 16 (4) | |||
| Long-term safety . | Total . | FC . | FCR . | |||
|---|---|---|---|---|---|---|
| Cases N (%) . | Patients N (%) . | Cases N (%) . | Patients N (%) . | Cases N (%) . | Patients N (%) . | |
| Total patients (safety population), N | 800 | 396 | 404 | |||
| Total cases [N (%)] and patients [N (%)] with ≥1 SPM | 136 (100) | 122 (15) | 77 (57) | 69 (17) | 59 (43) | 53 (13) |
| Secondary malignancies | ||||||
| Richter’s transformation | 38 (28) | 38 (5) | 25 (33) | 25 (6) | 13 (22) | 13 (3) |
| Solid tumors | 55 (40) | 52 (7) | 29 (38) | 28 (7) | 26 (44) | 24 (6) |
| Lung | 18/55 (33) | 18 (2) | 13/29 (45) | 13 (3) | 5/26 (20) | 5 (1) |
| Prostate | 8/55 (15) | 8 (1) | 2/29 (7) | 2 (1) | 6/26 (23) | 6 (2) |
| Renal/bladder | 7/55 (13) | 6 (1) | 3/29 (10) | 3 (1) | 4/26 (15) | 3 (1) |
| Colorectal | 2/55 (4) | 2 (<1) | 0/29 (0) | 0 (0) | 2/26 (8) | 2 (<1) |
| Melanoma | 8/55 (15) | 8 (1) | 3/29 (10) | 3 (1) | 5/26 (20) | 5 (1) |
| Breast | 3/55 (6) | 3 (<1) | 1/29 (3) | 1 (<1) | 2/26 (8) | 2 (<1) |
| Pancreatic | 2/55 (4) | 2 (<1) | 1/29 (3) | 1 (<1) | 1/26 (4) | 1 (<1) |
| Ovarian/uterine/cervical | 1/55 (2) | 1 (<1) | 0/29 (0) | 0 (0) | 1/26 (4) | 1 (<1) |
| Liver/gall bladder | 1/55 (2) | 1 (<1) | 1/29 (3) | 1 (<1) | 0/26 (0) | 0 (0) |
| Thyroid | 2/55 (4) | 2 (<1) | 2/29 (7) | 2 (1) | 0/26 (0) | 0 (0) |
| Pharyngeal/laryngeal | 1/55 (2) | 1 (<1) | 1/29 (3) | 1 (<1) | 0/26 (0) | 0 (0) |
| Other | 2/55 (4) | 2 (<1) | 2/29 (7) | 2 (1) | 0/26 (0) | 0 (0) |
| Hematologic neoplasia | 24 (18) | 23 (3) | 11 (14) | 11 (3) | 13 (22) | 12 (3) |
| AML/MDS | 14/24 (58) | 13 (2) | 7/11 (64) | 7 (2) | 7/13 (54) | 6 (2) |
| Indolent B-non-Hodgkin lymphoma | 3/24 (13) | 3 (<1) | 1/11 (9) | 1 (<1) | 2/13 (16) | 2 (<1) |
| Aggressive B-non-Hodgkin lymphoma | 2/24 (8) | 2 (<1) | 1/11 (9) | 1 (<1) | 1/13 (8) | 1 (<1) |
| ALL | 1/24 (4) | 1 (<1) | 0/11 (0) | 0 (0) | 1/13 (8) | 1 (<1) |
| CML | 1/24 (4) | 1 (<1) | 0/11 (0) | 0 (0) | 1/13 (8) | 1 (<1) |
| Other | 3/24 (13) | 3 (<1) | 2/11 (18) | 2 (<1) | 1/13 (8) | 1 (<1) |
| Basalioma, squamous cell | 19 (14) | 17 (2) | 12 (16) | 11 (3) | 7 (12) | 6 (2) |
| Prolonged neutropenia | ||||||
| 2 months after end of treatment | 101 (13) | 34 (9) | 67 (17) | |||
| 12 months after end of treatment | 30 (4) | 14 (4) | 16 (4) | |||
To determine the frequency of secondary malignancies, we retrospectively analyzed the data of the full safety population (N = 800). At a median observation time of 5.9 years, 136 cases of secondary malignancies were observed in 122 (15.3%) patients including 40.4% solid tumors (including melanoma; 55 cases), 27.9% Richter’s transformation (38 cases), 17.6% hematologic neoplasias (24 cases), and 14% other skin cancers like squamous cell basalioma (19 cases; Table 4). Of the hematologic neoplasias, 14 cases of MDS or AML were observed in 13 patients (6 [1.5%] for FCR and 7 [1.8%] for FC; P = .8) with a median time to onset of 39 and 40 months after last dose of study treatment with FC and FCR, respectively. Of these 13 patients, 6 (46.2%) had prolonged neutropenia at 12 months after treatment or thereafter (1 after FCR and 4 after FC treatment; P = .27). Further, there was no significant difference of the time to development of MDS/AML between both treatment arms (P = .98). Secondary malignancies including Richter’s transformation occurred in 53 (13.1%) patients after FCR and 69 (17.4%) patients after FC therapy (P = .1), with a median time to onset of <2 years after the start of treatment. The time to SPM did not differ significantly between the treatment arms. At 5 years after the start of treatment, 89.1% vs 83.2% of FCR- vs FC-treated patients were free of SPM (HR, 0.69; 95% CI, 0.47-1.00; P = .052). Richter’s transformations were observed twice as often in the FC arm (13 [3.2%] for FCR and 25 [6.3%] for FC; P = .046; Table 4). The standardized incidence ratio (SIR) for solid tumors of 1.02 (95% CI, 0.75-1.36) for the whole safety population showed no increased incidence in comparison with an age-matched general German population. The incidence of solid tumors was slightly increased in patients treated with FC (SIR, 1.14; 95% CI, 0.73-1.69), whereas patients treated with FCR experienced fewer solid tumors than expected (SIR, 0.91; 95% CI, 0.58-1.39), although neither of these achieved conventional statistical significance.
Discussion
With an extended observation time of almost 6 years, the CLL8 study continues to demonstrate a significant improvement in PFS and OS in physically fit patients treated with FCR compared with FC. Patients with del(17p) showed a significantly shorter OS than those in all the other cytogenetic subgroups. Conversely, of the patients with del(11q), considered an adverse prognostic group,17 patients with IGHV MUT responded very well to FCR and as a consequence showed an outcome similar to other [non-del(17p)] patients. Although the benefit of FCR was observed especially in OS of young individuals, it needs to be noted that the study was not designed to differentiate between CLL-related and CLL-unrelated deaths. Therefore, the competing risk was not analyzed.
FCR induced a higher rate of prolonged neutropenia during the first year after the end of treatment. To date, this prolonged neutropenia following FCR was not associated with an increased rate of MDS/AML.20,21 The frequency of secondary malignancies was similar in both arms. An increased frequency of Richter’s transformations was noted in the FC arm. These data are in line with the current literature.22,23 In addition, achievement of a response seemed to be correlated with a lower incidence of secondary malignancies and Richter’s transformations.24
In multivariate analyses including the most commonly used clinical and biological factors, several prognostic factors predicted OS. Each of these factors was shown to correlate with OS in previous publications.11,17,25-30 Because we observed a particularly good outcome of patients with IGHV MUT, we performed further analyses on the IGHV mutational status. The PFS in patients with IGHV MUT/UNM disease showed a significant difference in favor of the IGHV MUT subgroup. Most importantly, the IGHV MUT subgroup treated with FCR showed a significantly longer PFS and OS than those treated with FC. The median OS for patients with IGHV MUT CLL was not reached. More than 83% of the patients with IGHV MUT treated with FCR were still alive after almost 6 years of observation time. Notably, cytogenetic subgroup analysis of IGHV MUT patients treated with FCR confirmed this excellent outcome for all cytogenetic subgroups including del(11q), except for patients with del(17p) and normal karyotype. These patients also had a better prognostic index.26 Interestingly, IGHV mutational status did not impact on ORR, complete response, and MRD negativity.31
We therefore conclude that most patients with IGHV MUT CLL benefit substantially from FCR chemoimmunotherapy resulting in long-term control of the disease. This observed benefit of FCR for the majority of IGHV MUT patients should be considered in the design of future clinical trials. First, this finding may announce the emergence of a therapeutic approach that is guided by the IGHV mutational status. Second, the long-lasting PFS of a specific subgroup following first-line FCR therapy may render it more difficult to replace first-line chemoimmunotherapy by novel agents in this subset of patients with CLL. As a consequence, we need to test the value of new targeted therapies such as ibrutinib, idelalisib, obinutuzumab, and venetoclax against FCR in previously untreated, fit patients with CLL. For this purpose, many academic study groups have started phase 3 trials that systematically compare the potential of chemotherapy-free treatment strategies to FCR or other chemoimmunotherapies.32
Presented in part at the 54th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 10, 2012 and at the XVth International Workshop on CLL (iwCLL), September 10, 2013, Cologne, Germany.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients and their physicians for CLL8 trial participation and donation of samples, as well as Myriam Mendila, Nancy Valente, Stephan Zurfluh, Michael Wenger, Günter Fingerle-Rowson, Anne Westermann, Alana Hönig, and Jamie Wingate for support in conception and conduct of the trial.
This work was supported in part by the DFG Klinische Forschergruppe (KFO) 286 and funded by the Deutsche Forschungsgemeinschaft (grant HA 1680/14). Genetic analyses were supported by the Else Kröner-Fresenius-Stiftung (2012_A146), DFG (SFB 1074 project B2), and F. Hoffmann-La Roche.
Participating physicians are found in the supplemental Data.
Authorship
Contribution: K.F. designed the research, collected, analyzed, and interpreted the data, and wrote the paper; J.B. analyzed and interpreted the data and wrote the paper; A.M.F. collected, analyzed, and interpreted the data and wrote the paper; V.G. designed the research and wrote the paper; C.D.H. collected the data and wrote the paper; P.C. designed the research, collected, analyzed, and interpreted the data, and wrote the paper; P.L. collected the data and wrote the paper; J.v.T. analyzed and interpreted the data and wrote the paper; A.E. analyzed and interpreted the data and wrote the paper; C.M. collected, analyzed, and interpreted the data and wrote the paper; G.K. analyzed and interpreted the data and wrote the paper; M. Herling collected, analyzed, and interpreted the data and wrote the paper; E.T. collected the data and wrote the paper; K.-A.K. designed the research, collected, analyzed, and interpreted the data, and wrote the paper; B.E. designed the research, collected, analyzed, and interpreted the data, and wrote the paper. S.B. collected, analyzed, and interpreted the data and wrote the paper; J.F.S. collected, analyzed, and interpreted the data and wrote the paper; P.G. collected the data and wrote the paper; P.M. collected the data and wrote the paper; H.D. designed the research, analyzed, and interpreted the data and wrote the paper; M.K. designed the research, collected, analyzed, and interpreted the data, and wrote the paper; C.-M.W. analyzed and interpreted the data and wrote the paper; S.S. designed the research, collected, analyzed, and interpreted the data, and wrote the paper; and M. Hallek designed the research, collected, analyzed, and interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: A.M.F. receives honoraria from Roche and receives travel, accommodations, or expenses from Roche and Celgene. V.G. receives honoraria from Roche, Glaxo, and Bristol Myer Squibb, has a consulting or advisory role for Roche, is on the speakers' bureau for Roche, Mundipharma, Glaxo, and Bristol Myer Squibb, and receives travel, accommodations, or expenses from Roche. P.C. receives honoraria from Roche and Janssen, is on the speakers’ bureau for both, and receives travel, accommodations, or expenses from Astellas, Gilead, Janssen, Roche, and Mundipharma. P.L. receives honoraria from Janssen and receives travel, accommodations, or expenses from Janssen and Mundipharma. J.v.T. has a consulting or advisory role for Janssen and receives travel, accommodations, or expenses from Celgene. A.E. receives travel, accommodations, or expenses from Roche. M.H. has a patent or intellectual property interest. E.T. receives travel, accommodations, or expense from Glaxo Smith Kline. K.A.K. receives honoraria and research funding from Roche, has a consulting or advisory role, in on the speakers’ bureau, and receives travel, accommodations, or expenses from Roche. B.E. receives honoraria from Janssen, Gilead, AbbVie, GSK, Celgene, Roche, and Mundipharma and has a consulting or advisory role for Janssen, Gilead, AbbVie, GSK, Celgene, Roche, and Mundipharma. S.B. receives honoraria from Roche and AbbVie, research funding from Roche, AbbVie, and Celgene, has a consulting or advisory role for AbbVie and Roche, and receives travel, accommodations, or expenses from Roche. J.S. receives honoraria from Roche and has a consulting or advisory role, is on the speakers’ bureau, and receives travel, accommodations, or expenses for Roche. P.G. receives honoraria from AbbVie, Gilead, Janssen, Medimmune, and Pharmacyclics, research funding from Gilead, GSK, and Roche, has a consulting or advisory role for AbbVie, Gilead, Janssen, Medimmune and Pharmacyclics, and is on the speakers’ bureau for Gilead. P.M. receives honoraria from Gilead, Janssen, Takeda, Novartis, and Roche, has a consulting or advisory role for Gilead, Janssen, Takeda, Novartis, and Roche, and is on the speakers’ bureau for Roche. H.D. has a consulting or advisory role for Roche. M.K. receives honoraria from AbbVie, Gilead, Janssen, and Roche, research funding from Gilead, Roche, and Amgen, has a consulting or advisory role for AbbVie, Gilead, Janssen, and Roche, and receives travel, accommodations, or expenses from Roche, GSK, AbbVie, Gilead, and Janssen. C.M.W. receives honoraria and research funding from Roche and has a consulting or advisory role and receives travel, accommodations, or expenses from Roche. S.S. receives honoraria from Roche and has a consulting or advisory role and receives travel, accommodations, or expenses from Roche. M. Hallek receives honoraria from Janssen, Gilead, AbbVie, GSK, Celgene, Roche, and Mundipharma and has a consulting or advisory role for Janssen, Gilead, AbbVie, GSK, Celgene, Roche, and Mundipharma. The remaining authors declare no competing financial interests.
Correspondence: Kirsten Fischer, Department of Internal Medicine I, Center for Integrated Oncology, University of Cologne, Kerpener Strasse 62, 50924 Köln, Germany; e-mail: kirsten.fischer@uk-koeln.de.