Abstract
The discovery of the iron-regulatory hormone hepcidin in 2001 has revolutionized our understanding of iron disorders, and its measurement should advance diagnosis/treatment of these conditions. Although several assays have been developed, a gold standard is still lacking, and efforts toward harmonization are ongoing. Nevertheless, promising applications can already be glimpsed, ranging from the use of hepcidin levels for diagnosing iron-refractory iron deficiency anemia to global health applications such as guiding safe iron supplementation in developing countries with high infection burden.
Pathophysiological background
Hepcidin, a liver-derived peptide hormone, is a key regulator of systemic iron homeostasis, and its unbalanced production contributes to the pathogenesis of a spectrum of iron disorders. Hepcidin functions by blocking the following iron flows into plasma: duodenal absorption, release from macrophages recycling old red blood cells, and mobilization of stored iron from hepatocytes (for extensive reviews, see Ganz1 and Hentze et al2 ). This blocking of iron flows is achieved by hepcidin causing degradation of its receptor, the iron transporter ferroportin. Hepcidin production is tightly regulated: it is (1) increased by plasma and liver iron as a feedback mechanism to maintain stable body iron levels, (2) decreased by erythroid activity to ensure iron supply for erythropoiesis, and (3) increased by inflammation as a host defense mechanism to limit extracellular iron availability to microbes.1-3 Because hepcidin levels reflect the integration of multiple key signals involved in iron regulation, and hepcidin directly controls iron absorption and bioavailability in circulation,4-6 its measurement should be a useful clinical tool for the management of iron disorders. Although a thorough understanding of its unique advantages over traditional biomarkers of iron status in different conditions will require larger studies with head-to-head comparisons, we discuss here some studies already supporting the distinct utility of hepcidin measurements.
Hepcidin structure and kinetics
The bioactive circulating form of hepcidin is 25 amino acids in size. N-terminal degradation leads to smaller isoforms (hepcidin-24, -23, -22, and -20) of unknown significance.7-9 These isoforms are generally present in diseases with elevated hepcidin-25 levels, including chronic kidney disease and sepsis.10,11 Circulating hepcidin is bound to α-2-macroglobulin and albumin, but estimates of this binding vary from <3% to 89% of total.12,13 The impact of binding on hepcidin activity or assay performance is unclear. Hepcidin is rapidly excreted by the kidney and reabsorbed in the proximal tubules by megalin-dependent endocytosis, but urinary hepcidin levels generally correlate with serum levels,14,15 with the exception of renal diseases.16 Nevertheless, urinary hepcidin measurements may have utility in specific conditions, such as noninvasive measurement in children, screening in low-resource settings, or prediction of acute kidney injury.17
Preanalytical, analytical, and postanalytical aspects
The development of assays to quantify hepcidin in biological samples has proved challenging. Because hepcidin is a small, evolutionarily conserved peptide, it is difficult to generate antibodies for laboratory assays. Hepcidin quantification is further complicated by its tendency to aggregate and stick to laboratory plastics.18 Nonetheless, a number of well-performing assays have been established and are listed in supplemental Table 1, available on the Blood Web site. They are divided into two major groups: mass spectrometry–based and classical immunoassays.19 Mass spectrometry–based assays require relatively expensive equipment but are able to distinguish the hepcidin isoforms. Immunoassays generally lack specificity for hepcidin-25 and measure total hepcidin levels. The relevance of specifically measuring hepcidin-25 instead of total hepcidin for clinical decision making, however, is unclear.
In the absence of a primary reference material, a reference method, and a commutable calibrator, absolute hepcidin levels differ widely between assays (up to 10-fold).20 Studies aiming at harmonization are ongoing,21 but for now, these differences preclude data comparability and the establishment of a universal reference range. Rather, each method/laboratory needs to establish rigorous age- and sex-specific reference ranges, preferably not only for hepcidin but also for hepcidin-to-transferrin saturation and hepcidin-to-serum ferritin ratios. To date, only 2 large studies in Holland (n = 2998)22 and Italy (n = 1577)23 have evaluated serum hepcidin variations at the population level, clearly showing that hepcidin levels are lower in premenopausal vs postmenopausal women and are highly correlated with serum ferritin levels. In smaller studies, the within-subject variation of serum hepcidin was relatively high; hepcidin increased with prolonged fasting24 and showed both circadian rhythm and considerable (27%-50%) day-to-day variation.25 Hepcidin-25 values decrease within 1 to 2 days with storage at room temperature but are stable at 4°C, −20°C, and −80°C for at least 1 week, 4 weeks, and ∼2 years, respectively.8,26
General considerations for the clinical applications of hepcidin measurement
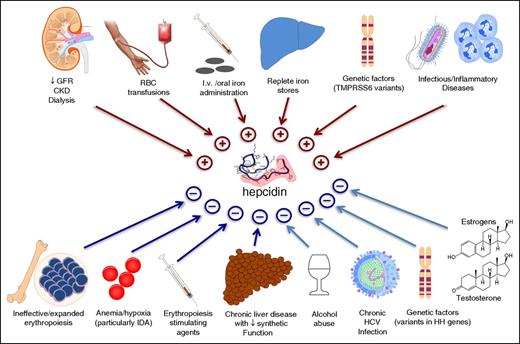
Like every hormone, hepcidin is under the influence of many different stimuli. Figure 1 summarizes the opposing effects exerted by a number of physiological and pathological conditions. Of note, the response is often quite rapid, making circulating hepcidin a very dynamic compartment. For example, hepcidin production increases substantially within a few hours after iron administration or inflammatory stimulation.15,28,29,33,34 Because many of the conditions listed in Figure 1 are common, several stimuli may be present simultaneously, with hepcidin output depending on the relative strength of each. For example, in severe ID, hepcidin production tends to be low, even in the presence of inflammation.46,47 Similarly, in conditions of ineffective/expanded erythropoiesis, like in non-transfusion-dependent thalassemias, signals released by bone marrow erythroid precursors tend to override those from replete iron stores.48 This results in relative hepcidin suppression in non-transfusion-dependent thalassemias,27,49,50 other iron-loading anemias,3,51 and even in β-thalassemia trait.50 One such erythroid signal, erythroferrone, has been recently identified in a mouse model.52 In line with this pathophysiological model, serum hepcidin fluctuates in transfusion-dependent β-thalassemia, with levels increasing soon after red blood cell transfusions (which suppress ineffective erythropoiesis) and declining during intertransfusional periods.53 Because of the highly dynamic and multifactorial regulation, a key practical message is that, in a given individual, the correct interpretation of hepcidin levels requires accurate knowledge of the overall clinical context (Table 18,19,22,23,25,26 )
Clinical conditions known to influence circulating hepcidin levels. Clinically relevant conditions include CKD,11,16 RBC transfusions,27 iron administration,28.29 replete iron stores,1 TMPRSS6 variants,30,31 infections/inflammatory disorders,32-34 ineffective erythropoiesis,3,49 hypoxia,35,36 administration of erythropoietic stimulating agents,37 chronic liver diseases,38 alcohol abuse,39 HCV,40 hemochromatosis-related mutations,1,28,41,42 and administration of the sex hormones testosterone43 and estrogens.44,45 CKD, chronic kidney disease; GFR, glomerular filtration rate; HCV, hepatitis C virus; HH, hereditary hemochromatosis; IDA, iron deficiency anemia; RBC, red blood cell; TMPRSS6 (transmembrane protease serine 6), the gene encoding for matriptase-2.
Clinical conditions known to influence circulating hepcidin levels. Clinically relevant conditions include CKD,11,16 RBC transfusions,27 iron administration,28.29 replete iron stores,1 TMPRSS6 variants,30,31 infections/inflammatory disorders,32-34 ineffective erythropoiesis,3,49 hypoxia,35,36 administration of erythropoietic stimulating agents,37 chronic liver diseases,38 alcohol abuse,39 HCV,40 hemochromatosis-related mutations,1,28,41,42 and administration of the sex hormones testosterone43 and estrogens.44,45 CKD, chronic kidney disease; GFR, glomerular filtration rate; HCV, hepatitis C virus; HH, hereditary hemochromatosis; IDA, iron deficiency anemia; RBC, red blood cell; TMPRSS6 (transmembrane protease serine 6), the gene encoding for matriptase-2.
Hepcidin measurement in clinical practice: a decalogue for the hematologist
| . | Comments . | Reference . |
|---|---|---|
| Checklist before ordering the assay | ||
| 1. Ensure local availability of a validated assay | See text and supplemental Table 1 | 19 |
| 2. Ensure control of preanalytical conditions (including diurnal rhythm) | See text | 8, 25, 26 |
| 3. Refer to age- and sex-specific ranges | Significant differences between males and females, particularly during fertile period | 22, 23 |
| 4. Interpret hepcidin value into a minimum laboratory context (CBC, ferritin, transferrin saturation, CRP, serum creatinine, and liver function tests) | See Figure 1 | — |
| 5. Be aware of any potential confounders/comorbidities in the individual patient | See Figure 1 | — |
| Most promising applications | ||
| 6. Evaluation of suspected IRIDA | Virtually diagnostic in an appropriate clinical context | 54, 55 |
| 7. Evaluation of IO disorders | For example, ferroportin disease due to hepcidin resistant mutations (see text) | 41, 42, 49, 51, 56, 57 |
| 8. Diagnosis of concomitant ID in patients with ACD | Promising reports in rheumatoid arthritis and inflammatory bowel disease patients, and in African children | 32, 58-60 |
| 9. Guide for iron therapy | For example, selection of patients for direct IV supplementation; oral administration in children from developing countries with high prevalence of infectious diseases (see text) | 6, 32, 58, 61-63 |
| 10. Monitoring of treatments targeting the hepcidin/ferroportin axis | To be confirmed by further studies | 64 |
| . | Comments . | Reference . |
|---|---|---|
| Checklist before ordering the assay | ||
| 1. Ensure local availability of a validated assay | See text and supplemental Table 1 | 19 |
| 2. Ensure control of preanalytical conditions (including diurnal rhythm) | See text | 8, 25, 26 |
| 3. Refer to age- and sex-specific ranges | Significant differences between males and females, particularly during fertile period | 22, 23 |
| 4. Interpret hepcidin value into a minimum laboratory context (CBC, ferritin, transferrin saturation, CRP, serum creatinine, and liver function tests) | See Figure 1 | — |
| 5. Be aware of any potential confounders/comorbidities in the individual patient | See Figure 1 | — |
| Most promising applications | ||
| 6. Evaluation of suspected IRIDA | Virtually diagnostic in an appropriate clinical context | 54, 55 |
| 7. Evaluation of IO disorders | For example, ferroportin disease due to hepcidin resistant mutations (see text) | 41, 42, 49, 51, 56, 57 |
| 8. Diagnosis of concomitant ID in patients with ACD | Promising reports in rheumatoid arthritis and inflammatory bowel disease patients, and in African children | 32, 58-60 |
| 9. Guide for iron therapy | For example, selection of patients for direct IV supplementation; oral administration in children from developing countries with high prevalence of infectious diseases (see text) | 6, 32, 58, 61-63 |
| 10. Monitoring of treatments targeting the hepcidin/ferroportin axis | To be confirmed by further studies | 64 |
ACD, anemia of chronic disease; CBC, complete blood count; CRP, C-reactive protein; ID, iron deficiency; IO, iron overload; IRIDA, iron-refractory iron deficiency anemia.
Most promising applications of hepcidin measurements in hematology
Diagnosis of IRIDA
This genetic disease, due to mutations in the hepcidin inhibitor TMPRSS6 (encoding matriptase-2),30 is characterized by IDA with inappropriately normal or high hepcidin levels.54,55 Thus, oral iron is ineffective and parenteral administration is needed to achieve at least a partial response. In classical IDA, hepcidin is suppressed below the limit of detection in biological fluids.15,19 By contrast, detection of pseudo-normal/elevated serum hepcidin in an appropriate clinical context can be considered virtually diagnostic of IRIDA, even without confirmation by TMPRSS6 sequencing (eg, in a young patient with unexplained IDA not responding to oral iron and with positive family history). Measurement of hepcidin may also help in the diagnosis of other atypical microcytic anemias due to rare genetic disorders of iron metabolism or heme synthesis (reviewed in Donker et al55 ), although data are presently insufficient (Table 1).
Diagnosis of IO disorders
Classical type 1 (HFE-related) HH is by far the commonest genetic disorder leading to IO in populations of northern European descent,65 but in other ethnicities, “atypical” IO accounts for up to 35% to 40% of cases.66 In type 1 HH at diagnosis, serum hepcidin levels are typically in the low to normal range and inappropriate for the degree of IO (manifesting as a low hepcidin-to-ferritin ratio).41 Hepcidin is further suppressed after normalization of iron stores by phlebotomies15,41 and shows a blunted response to oral iron.28 This blunted response is also seen in type 3 (TFR2-related) HH,28 whereas in type 2A/B or “juvenile” hemochromatosis (HJV or HAMP related, respectively), hepcidin levels are markedly suppressed/undetectable.19,42 By contrast, in type 4B HH or atypical ferroportin disease due to mutations in SLC40A1 conferring partial or complete resistance to hepcidin,67 serum hepcidin levels are substantially increased.56 Some data support using hepcidin measurement as a guide for the follow-up genetic testing of IO patients, particularly in populations in whom classical type 1 HFE-related HH is rare.57 Furthermore, during treatment of type 1 HH, monitoring hepcidin to prevent its complete suppression may curb iron hyperabsorption in the maintenance phase, possibly decreasing the need for phlebotomies. In iron-loading anemias, hepcidin measurement may also be valuable for identifying the most severely affected patients, helping to predict the development of IO and guiding the therapy.
Diagnosis and management of IDA
In IDA, hepcidin levels are generally suppressed to allow maximal iron absorption,15,19 but some patients, particularly the elderly,61 may have detectable hepcidin levels because of comorbidities like renal, inflammatory, or neoplastic diseases. Because hepcidin directly controls iron absorption,4,5 serum hormone levels have the potential to predict poor responsiveness to oral iron, preventing possible detrimental effects of oral iron on the gut microbiome and metabolome68 and eliminating delays before switching to IV iron. The usefulness of measuring basal hepcidin to personalize the optimal route of iron administration has been recently showed in patients with IDA,62 chronic rheumatic anemia,32 and chemotherapy-associated anemia.63 However, large prospective trials are needed to confirm this attractive hypothesis. Hepcidin has also proved effective for detecting ID in blood donors,69 the prevention of which is a major concern for blood services.
Notably, determination of serum hepcidin may help to solve a major global health problem; that is, the appropriateness of oral iron supplementation in children from regions with high infection burden. ID is highly prevalent in these regions and affects children’s physical growth and cognitive performance.70 Nonetheless, systematic iron supplementation has been associated with serious adverse outcomes, including increased mortality.71-73 Such detrimental effects have been mainly attributed to increased vulnerability to malaria and microbial agents, which are engaged in the host–pathogen battle for essential iron.68,74,75 Hepcidin capability to integrate competing signals (anemia, ID, and infection) makes its measurement promising in this setting. Indeed, 2 recent elegant studies using iron isotopes showed that low serum hepcidin was a good predictor of erythrocyte iron incorporation in African children, and suggest hepcidin as a guide for distinguishing individuals “ready to receive iron” from those in whom it should be avoided.6,58 This fascinating hypothesis, requiring the development of a validated point-of-care hepcidin assay, is actively under evaluation. However, because iron-induced reticulocytosis could increase susceptibility to malaria,76 and low hepcidin values do not necessarily exclude a concurrent infection,46 safe iron supplementation in malaria-endemic areas should entail not only the ability to absorb and incorporate iron but also effective measures to prevent exacerbations of infections.
Distinction between ID and ACD
ACD is a common condition with complex pathogenesis.77 Hepcidin-driven iron maldistribution plays a substantial role because iron is trapped in macrophages and less available for erythropoiesis. Patients with chronic inflammatory disorders are also at risk of developing ID. For example, occult/overt blood loss frequently occurs in conditions like inflammatory bowel diseases, chronic hemodialysis, and concomitant use of nonsteroidal anti-inflammatory drugs or antithrombotic drugs. In such settings, ID is difficult to detect using traditional iron biomarkers,78 but hepcidin may be helpful. Patients with inflammatory disorders and concomitant ID typically have lower hepcidin levels as compared with those with “pure” ACD.59 Indeed, hepcidin has proved effective in distinguishing IDA from ACD in patients with rheumatoid arthritis,32 inflammatory bowel diseases,60 cancer-related anemia,79 and critical illnesses,47 as well as in African children.58 This, again, can help in personalizing iron therapy to avoid delays or unnecessary/harmful treatment. However, because of the highly variable cutoff reported with different assays, harmonization of hepcidin assays is necessary before giving practical recommendations. Supplemental Table 2 shows examples of the diagnostic performance of hepcidin (eg, area under the curve of receiver operating characteristic, sensitivity, and specificity) in different clinical settings.
Companion diagnostic for novel therapies
Hepcidin discovery has opened unprecedented therapeutic opportunities for iron disorders. Hepcidin pharmacology is an active field with a number of drugs in the pipeline, including hepcidin agonists for the treatment of IO, and hepcidin antagonists for the treatment of iron restriction in ACD (reviewed in Ruchala and Nemeth64 and Camaschella80 ). Measuring hepcidin levels is anticipated to be helpful both in selecting patients and in monitoring the effects of novel targeted therapies.
Conclusions
Hepcidin is a promising tool to be added to the present battery of diagnostic tests for iron status, especially in ID, because it has the potential to differentiate from ACD and to inform about the ability to respond to oral iron. However, full inclusion in clinical practice and public health requires further efforts to harmonize the assays, assess the relevance of measuring specific hepcidin isoforms, define clinical decision limits, and make validated assays universally available.
The online version of this article contains a data supplement.
Authorship
Contribution: D.G., E.N., and D.W.S. conceived of and cowrote the manuscript.
Conflict-of-interest disclosure: D.W.S. is an employee of Radboud University Medical Center, which offers high-quality hepcidin measurements to the medical, scientific, and pharmaceutical communities via the www.hepcidinanalysis.com initiative on a fee-for-service basis. E.N. is a consultant and stock holder for Intrinsic LifeSciences, Merganser Biotech, and Silarus Therapeutics. D.G. declares no competing financial interests.
Correspondence: Domenico Girelli, Department of Medicine, University of Verona, Veneto Region Referral Center for Iron Metabolism Disorders, Azienda Ospedaliera Universitaria Integrata Verona, Policlinico G.B. Rossi, 37134 Verona, Italy; e-mail: domenico.girelli@univr.it; and Dorine W. Swinkels, Department of Laboratory Medicine, Translational Metabolic Laboratory 830, Radboud University Medical Center, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: dorine.swinkels@radboudumc.nl.