In this issue of Blood, Aguilar-Hernandez et al1 refine our understanding of the mechanism of action of interleukin 4 (IL-4) in promoting B-cell receptor (BCR)-mediated signaling in primary human chronic lymphocytic leukemia (CLL) and resistance to a Bruton tyrosine kinase inhibitor (BTKi), ibrutinib and a phosphatidylinositol 3-kinase δ inhibitor (PI3Kδi), idelalisib. The tumor microenvironment is firmly established as providing stimuli to cause CLL cells residing in the lymph nodes or bone marrow to proliferate, survive, and resist chemotherapy as demonstrated by animal models, which suggests a requirement for T cells2 and stromal cells,3 whereas immunohistochemistry has shown close connections between leukemic B cells and T cells in lymph node proliferation centers. As well as direct cell:cell contacts, growth factors, including IL-4, and chemokines are also important modes of communication between the microenvironment and the leukemic cell. Many of these soluble factors are produced in T cells, and the CD4+ T-cell subset, the follicular helper T-cell subset, and TH2 cells may be particularly important in producing IL-4.
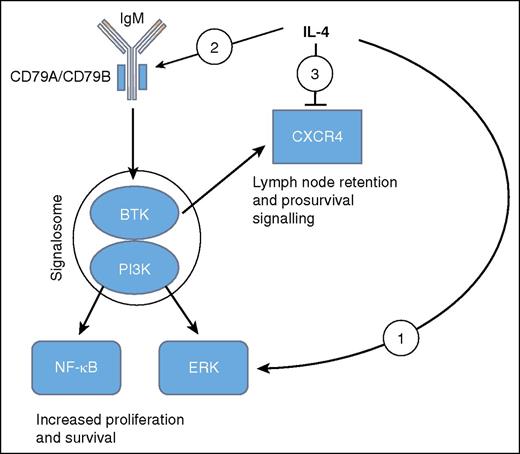
Diagrammatic representation of the effects of IL-4 as elucidated by Aguilar-Hernandez et al and others.5,6,8 Overall BCR signaling in CLL acts to increase proliferation and survival, whereas CXCR4 appears to mediate lymph node retention as well as survival. (1) IL-4 promotes a BCR pathway to ERK but not NF-κB. (2) IL-4 induces CD79A and CD79B and increases surface IgM. (3) IL-4 represses CXCR4.
Diagrammatic representation of the effects of IL-4 as elucidated by Aguilar-Hernandez et al and others.5,6,8 Overall BCR signaling in CLL acts to increase proliferation and survival, whereas CXCR4 appears to mediate lymph node retention as well as survival. (1) IL-4 promotes a BCR pathway to ERK but not NF-κB. (2) IL-4 induces CD79A and CD79B and increases surface IgM. (3) IL-4 represses CXCR4.
IL-4 is an important factor driving normal B-cell proliferation and class switching.4 Work on the mechanism of action of IL-4 has shown that it modifies BCR signaling: in the absence of IL-4, BCR signals phosphorylate ERK in a PI3K, phospholipase C, and protein kinase Cβ–dependent manner,5 these enzymes constituting essential components of the signalosome. However, the presence of IL-4 alters BCR signaling in such a way that ERK phosphorylation is no longer dependent on the signalosome elements.5 In addition, although the BCR pathway activates NF-κB through the signalosome, the IL-4 pathway leads to ERK activation without affecting NF-κB. Thus a model has emerged, mainly from studies of mouse B cells, of parallel BCR pathways—one arm mediated by the BCR in the absence of IL-4 and dependent on the signalosome and the other, in the presence of IL-4, leading to ERK phosphorylation without requiring the signalosome.
More recent work using normal mouse B cells has further defined the mechanism of action by showing that IL-4 induces the proteins Igα and Igβ that form a sheath around the transmembrane portion of surface IgM, thereby increasing overall surface IgM expression and increasing B-cell activation and proliferation.6
Aguilar-Hernandez et al add to this data by demonstrating apparently similar mechanisms in primary human CLL: they show that IL-4 increases the surface expression of IgM together with that of the human sheath proteins, CD79A and CD79B especially on CLL with unmutated immunoglobulin genes (U-CLL). Interestingly, this alteration might be expected to enhance both the IL-4–dependent and –independent components of BCR signaling in U-CLL, thus providing an additional boost to pathways that are already overactive compared with mutated CLL (M-CLL). In an additional line of work, these authors show that IL-4 reduced expression of the chemokine receptor CXCR4 on the surface of the human leukemic cells. CXCR4 engagement by its ligand CXCL12 produces prosurvival effects in human CLL,7 and more recently, abrogating BCR signaling with a BTK inhibitor in a mouse model has been shown to reduce expression and function of CXCR4 to lead to reduced retention of leukemic cells in tissue.8 Therefore, CXCR4 has been implicated in survival and trafficking of CLL. It will clearly take more work to arrive at an overall view of the effects of IL-4 in CLL but Aguilar-Hernandez et al demonstrate how this growth factor modifies signaling in multiple pathways (see figure).
What are the implications of this work for the clinical use of small-molecule inhibitors in CLL? Inhibitors for BTK and PI3Kδ are available and in clinical use. BTKi, in particular, are well-tolerated and their effects appear to be sustained over several years. Whether the addition of other small-molecule inhibitors to a BTKi, to deepen responses, would be clinically beneficial if it could be accomplished without an increase in toxicity, remains an open question. Aguilar-Hernandez et al demonstrate that IL-4 suppresses the effects of a BTKi and a PI3Kδi on BCR signaling and protects against apoptosis. The effects of IL-4 on CXCR4, however, would appear to suggest that it might reduce the prosurvival effects of CXCL12 signaling. Small-molecule inhibitors and blocking antibodies9 against CXCR4 are available, and others have raised the possibility that CXCR4 might be a therapeutic target in CLL.10 Intriguingly, the results of Aguilar-Hernandez et al might suggest that the combination of interrupting BCR signaling and CXCR4 signaling could be a useful therapeutic route.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal