Key Points
AD STAT1 GOF is the most common genetic cause of inherited CMC and is not restricted to a specific age or ethnic group.
STAT1 GOF underlies a variety of infectious and autoimmune features, as well as carcinomas and aneurysms associated with a poor outcome.
Abstract
Since their discovery in patients with autosomal dominant (AD) chronic mucocutaneous candidiasis (CMC) in 2011, heterozygous STAT1 gain-of-function (GOF) mutations have increasingly been identified worldwide. The clinical spectrum associated with them needed to be delineated. We enrolled 274 patients from 167 kindreds originating from 40 countries from 5 continents. Demographic data, clinical features, immunological parameters, treatment, and outcome were recorded. The median age of the 274 patients was 22 years (range, 1-71 years); 98% of them had CMC, with a median age at onset of 1 year (range, 0-24 years). Patients often displayed bacterial (74%) infections, mostly because of Staphylococcus aureus (36%), including the respiratory tract and the skin in 47% and 28% of patients, respectively, and viral (38%) infections, mostly because of Herpesviridae (83%) and affecting the skin in 32% of patients. Invasive fungal infections (10%), mostly caused by Candida spp. (29%), and mycobacterial disease (6%) caused by Mycobacterium tuberculosis, environmental mycobacteria, or Bacille Calmette-Guérin vaccines were less common. Many patients had autoimmune manifestations (37%), including hypothyroidism (22%), type 1 diabetes (4%), blood cytopenia (4%), and systemic lupus erythematosus (2%). Invasive infections (25%), cerebral aneurysms (6%), and cancers (6%) were the strongest predictors of poor outcome. CMC persisted in 39% of the 202 patients receiving prolonged antifungal treatment. Circulating interleukin-17A–producing T-cell count was low for most (82%) but not all of the patients tested. STAT1 GOF mutations underlie AD CMC, as well as an unexpectedly wide range of other clinical features, including not only a variety of infectious and autoimmune diseases, but also cerebral aneurysms and carcinomas that confer a poor prognosis.
Introduction
Chronic mucocutaneous candidiasis (CMC), characterized by persistent or recurrent infections of the nails, skin, oral or genital mucosae with Candida spp., mainly C albicans, may result from primary immunodeficiencies (PIDs).1 In patients with T-cell deficiencies, CMC is associated with various infections and autoimmune diseases.1 CMC is one of the most common infections in patients with autosomal dominant (AD) hyper–immunoglobulin E (IgE) syndrome (AD-HIES)2 and the only infectious complication reported in patients with autosomal recessive (AR) autoimmune polyendocrine syndrome type I (APS-I).3 By contrast, since the late 1960s, patients have been reported who display an apparently inherited form of isolated CMC and no distinctive associated clinical or immunological phenotype (CMC disease, CMCD).1 However, invasive candidiasis, peripheral dermatophytosis, bacterial infections of the skin, and respiratory tract have occasionally been reported in some CMCD patients.1,4 Autoimmune diseases, typically affecting the thyroid, have also been described in these patients.1 CMCD can be life-threatening as it is associated with an unexplained increase in the risk for squamous cell carcinoma1,5 and cerebral aneurysm.1,6 A number of the CMCD cases reported belonged to multiplex and/or consanguineous families suggestive of AD and AR inheritances.1
The pathogenesis of CMC, first studied in PIDs with syndromic CMC, strongly suggested a role for interleukin 17A (IL-17A), IL-17F, and IL-22 in mucocutaneous immunity to Calbicans.1 Patients with AD HIES, heterozygous for STAT3 loss-of-function (LOF) mutations, have low proportions of IL-17A, IL-17F, and IL-22-producing T cells ex vivo and in vitro.7-9 Patients with AR APS-1 and biallelic LOF mutations of AIRE10 have high plasma titers of neutralizing autoantibodies against IL-17A, IL-17F, and IL-22.11,12 Low proportions of circulating IL-17A, IL-17F, and IL-22-producing T cells have also been found in some patients with Mendelian susceptibility to mycobacterial disease and AR IL-12 receptor β1 or IL-12p40 deficiency, who may also develop CMC.8,13 Patients with AR caspase recruitment domain-containing protein 9 (CARD9) deficiency and various invasive fungal diseases occasionally display CMC and low IL-17A producing T-cell counts.4,14,15 These findings paved the way for the candidate gene-based discovery of AR IL-17 receptor A (IL-17RA), AR IL-17RC, AR ACT1, and AD IL-17F deficiencies in some patients with inherited CMCD.1,16-18 However, the genetic etiology of CMCD remained unknown for most patients, including those with low proportions of circulating IL-17A and IL-17F T cells.19
Genome-wide strategies have identified heterozygous STAT1 gain-of-function (GOF) mutations20,21 as the underlying cause in about half of CMCD patients (depending on the centers). Among the 400 CMCD patients studied in the laboratory of the corresponding author (Necker and Rockefeller branches), approximately half of them carry STAT1 GOF mutations (A. Puel, unpublished data). These mutations affect the coiled-coil domain or DNA-binding domain of STAT1. They increase STAT1 phosphorylation by impairing nuclear dephosphorylation and are GOF for the STAT1-dependent cytokines interferon α/β (IFN-α/β), IFN-γ, and IL-27, and STAT3-dependent IL-6 and IL-21.20,22-24 Impaired IL-17A- and/or IL-17F-producing T-cell development accounts, at least in part, for CMCD, but the underlying mechanisms remain elusive. Since 2011, 184 patients from 120 kindreds with STAT1 GOF mutations have been described.20,21,23-59 Most reports have focused on the molecular and cellular defects of 1 or a small series of patients. This provides useful but incomplete clinical information. The comprehensive clinical features and outcomes of patients with STAT1 GOF mutations remain undefined. We therefore undertook the detailed clinical analysis of an international cohort of 274 patients with genetically and biochemically confirmed STAT1 GOF mutations. We collected information concerning demographic and clinical features, clinical outcome, preventive and curative treatments, and immunological and hematological investigations.
Methods
Study design, ethical concerns, definitions and methods for data collection, immunological and genetic analyses, as well as statistical analysis are described in the supplemental Appendix (available on the Blood Web site).
Results
Genetic and immunological features
We studied 274 patients from 167 kindreds originating from 40 countries on 5 continents, most of whom were from Europe (62%) (supplemental Table 1). Male/female (M/F) ratio was 1.03, and median age of the patients at the time of the study was 22 years (range: 1-71 years). In total, 167 of the 274 cases (61%) were familial (60 kindreds) (supplemental Table 1), and the remaining 107 cases being sporadic (107 kindreds). Three of the sporadic cases had a relevant familial history of disease previously reported to be associated with CMCD (carcinoma, autoimmunity, or aneurysm) that could not be explored genetically. We identified 76 mutations in STAT1 (supplemental Table 1); the mutation affected the coiled-coil domain in 104 kindreds (62%), the DNA-binding domain in 58 (35%) kindreds, the transactivation domain in 2 (1%) kindreds, the N-terminal domain in 2 (1%) kindreds, and the SH2 domain in 1 (1%) kindred. All the 76 STAT1 mutations were tested in vitro and were all found to be GOF in each functional assay tested (at least 1 assay per mutation) (S.O., J.-L.C. and A.P., unpublished data).20,21,23,30,33,35,43,44,46-48 Blood leukocyte subsets were analyzed in 232 patients (Table 1). Most patients did not display diagnostically relevant functional defects in immune parameters except low memory B cells (49% of the 53 patients tested) as well as low IgG2 (38%) or IgG4 (50%) levels. Abnormal immunological features were significantly associated with lower respiratory tract infections (LRIs) (ie, low CD19+ [P = .02] or CD4+ [P = .005] cell subsets), viral infections (low proportions of CD19+ [P < .001] or CD4+ [P = .009] cell subsets), or mycobacterial infections (low proportions of CD19+ [P = .005], or CD4+ [P = .005] cell subsets, low IgG levels [P < .001] without or with weak antibody [Ab] production to protein antigens [P < .001]). Regarding Th17 immunity, lower proportions than normal of ex vivo IL-17A-producing T cells and of IL-17A production, measured after 12 hours of stimulation with phorbol 12-myristate 13-acetate (PMA)–ionomycin or Candida spp.,60 were observed in 82% of 49 patients and 40% of 10 patients tested, respectively. Further details regarding genetic and immunological features are described in the supplemental Appendix.
Immunological investigations of patients with STAT1 GOF mutations
| Biological investigations . | Patients tested (n) . | Normal (%) . | Low (%) . | High (%) . |
|---|---|---|---|---|
| Total lymphocyte rate | 232 | 81 | 19 | — |
| CD4+ T lymphocytes | 222 | 72 | 28 | — |
| CD8+ T lymphocytes | 222 | 83 | 16 | 1 |
| CD19+ or CD20+ B lymphocytes | 209 | 81 | 19 | — |
| CD27+CD19+ memory B lymphocytes | 53 | 51 | 49 | — |
| CD16+CD56+ NK cells | 158 | 73 | 25 | 2 |
| CD3+IL-17+ or CD4+IL-17+ T lymphocytes* | 49 | 18 | 82 | — |
| CD4+IFNγ+ T lymphocytes† | 13 | 69 | 31 | — |
| T-cell proliferation (mitogen and/or antigen) | 119 | 68 | 32 | — |
| PBMC IL-17 production (pg/mL)‡ | 10 | 60 | 40 | — |
| IgG levels (g/L) | 229 | 77 | 3 | 20§ |
| IgA levels (g/L) | 219 | 82 | 14 | 4 |
| IgM levels (g/L) | 221 | 100 | — | — |
| IgG1 levels (g/L) | 94 | 100 | — | — |
| IgG2 levels (g/L) | 94 | 62 | 38 | — |
| IgG4 levels (g/L) | 94 | 50 | 50 | — |
| IgE levels (kIU/L) | 125 | 97 | — | 3|| |
| Antibodies against protein antigens¶ | 111 | 77 | 23 | — |
| Biological investigations . | Patients tested (n) . | Normal (%) . | Low (%) . | High (%) . |
|---|---|---|---|---|
| Total lymphocyte rate | 232 | 81 | 19 | — |
| CD4+ T lymphocytes | 222 | 72 | 28 | — |
| CD8+ T lymphocytes | 222 | 83 | 16 | 1 |
| CD19+ or CD20+ B lymphocytes | 209 | 81 | 19 | — |
| CD27+CD19+ memory B lymphocytes | 53 | 51 | 49 | — |
| CD16+CD56+ NK cells | 158 | 73 | 25 | 2 |
| CD3+IL-17+ or CD4+IL-17+ T lymphocytes* | 49 | 18 | 82 | — |
| CD4+IFNγ+ T lymphocytes† | 13 | 69 | 31 | — |
| T-cell proliferation (mitogen and/or antigen) | 119 | 68 | 32 | — |
| PBMC IL-17 production (pg/mL)‡ | 10 | 60 | 40 | — |
| IgG levels (g/L) | 229 | 77 | 3 | 20§ |
| IgA levels (g/L) | 219 | 82 | 14 | 4 |
| IgM levels (g/L) | 221 | 100 | — | — |
| IgG1 levels (g/L) | 94 | 100 | — | — |
| IgG2 levels (g/L) | 94 | 62 | 38 | — |
| IgG4 levels (g/L) | 94 | 50 | 50 | — |
| IgE levels (kIU/L) | 125 | 97 | — | 3|| |
| Antibodies against protein antigens¶ | 111 | 77 | 23 | — |
PBMC, peripheral blood mononuclear cell.
CD3+IL17+ lymphocyte normal range (% of CD3+): 0.7-1.7; CD4+IL17+ lymphocyte normal range (% of CD4+): 6-22.60
CD4+IFNγ+ lymphocytes normal range (%): 6-18.60
IL-17 (PBMC stimulated with Candida) normal range: 900-5000 pg/mL.60
IgG >15 g/dL.
IgE >140 kIU/L.
Tetanus, diphtheria toxoid, or poliovirus.
Fungal infections
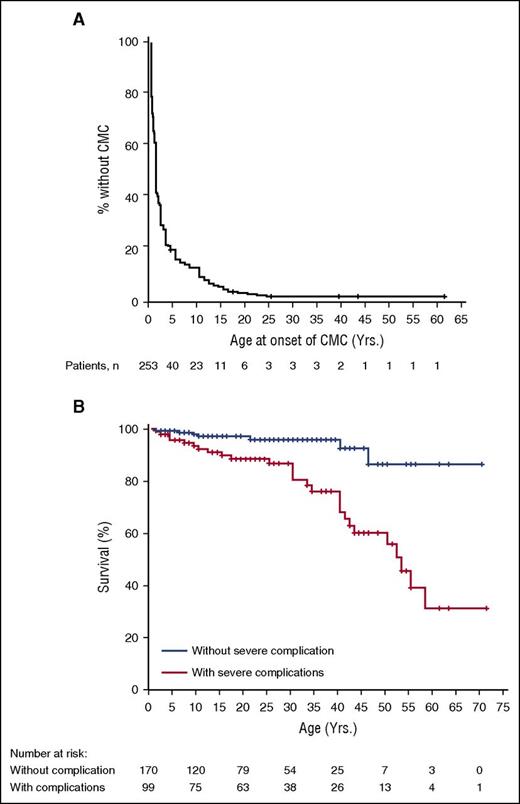
CMC was observed in 268 (98%) of the 274 individuals carrying STAT1 GOF mutations (supplemental Table 2), with a median age at onset of 1 year (range: birth to 24 years) (Figure 1A). None of the 6 patients without CMC (median age 32 years, range: 4-61 years) was totally asymptomatic. One had invasive fungal infection, 5 had invasive bacterial infections, 4 had hypothyroidism, and 1 had a cerebral aneurysm. Three of them had a familial history of CMC. Mucocutaneous fungal infections mostly affected the oral mucosae (93% of patients, thrush, glossitis, and/or cheilitis) (Table 2). Skin (57%, pustules, annular plaques, intertrigo), esophageal/genital (56%), and/or nail (56%, onyxis, perionyxis) infections were also often reported (Table 2). Recurrent and/or severe aphthous stomatitis occurred in 125 (46%) patients, and candidiasis of the scalp occurred in 55 (20%) patients. Candida spp. were isolated from various specimens (skin, nails, throat, genital/esophageal mucosae) (Table 3), and C albicans was the species most frequently isolated (95% of the 147 Candida isolates). Superficial dermatophytic infections of the scalp, skin or nails were suspected in 44 (16%) patients and were microbiologically confirmed (Trichophyton spp., Microsporon spp.) in 52% of these patients. Photographs of mucocutaneous fungal infections are found in Figure 2. Twenty-eight (10%) patients developed invasive fungal infections (Table 2). Ten patients suffered from invasive candidiasis, and fungal pneumonia was observed in 17 patients (Pneumocystis jirovecii [n = 6], Aspergillus spp. [n = 5], Cryptococcus spp. [n = 4], and Histoplasma spp. [n = 2]). Two patients had fungal nephritis (C curvatus and Trichosporon asahii), 2 patients suffered from cryptococcal meningitis, and 3 patients displayed disseminated fungal disease (Coccidioides spp. or Mucoraceae) (Table 3). Overall, although most patients had CMC, a substantial number (24%, cumulative) displayed various other superficial (17%) or invasive (10%) fungal diseases.
Kaplan-Meier curves for onset of CMC and outcome. (A) Onset of CMC. Exact age at onset of CMC was available for 253 patients. (B) Overall survival curves. Age at the time of the study was available for 269 patients. Patients with severe complications (ie, patients who displayed invasive infections, aneurysms, and/or tumors) were compared with patients without any severe complication (log-rank test, P < .001).
Kaplan-Meier curves for onset of CMC and outcome. (A) Onset of CMC. Exact age at onset of CMC was available for 253 patients. (B) Overall survival curves. Age at the time of the study was available for 269 patients. Patients with severe complications (ie, patients who displayed invasive infections, aneurysms, and/or tumors) were compared with patients without any severe complication (log-rank test, P < .001).
Sites of infection in patients with STAT1 GOF mutations
| Type of infections . | Patients (%) . |
|---|---|
| n = 274 . | |
| Mucocutaneous fungal infections | 268 (98) |
| Oropharyngeal mycosis | 254 (93) |
| Cutaneous mycosis | 155 (57) |
| Esophageal/genital mycosis | 153 (56) |
| Onychomycosis | 153 (56) |
| Aphtous stomatitis | 125 (46) |
| Scalp mycosis | 55 (20) |
| Invasive fungal infections | 28 (10) |
| Invasive candidiasis | 10 (4) |
| Other invasive infections | 20 (7) |
| Bacterial infections* | 202 (74) |
| LRI | 129 (47) |
| ENT | 121 (44) |
| Skin | 77 (28) |
| Others† | 24 (9) |
| Mycobacterial infections | 17 (6) |
| Lung disease | 6 (2) |
| Adenitis/skin disease | 5 (2) |
| Disseminated disease | 6 (2) |
| Viral infections* | 103 (38) |
| Cutaneous | 88 (32) |
| Systemic | 23 (8) |
| Type of infections . | Patients (%) . |
|---|---|
| n = 274 . | |
| Mucocutaneous fungal infections | 268 (98) |
| Oropharyngeal mycosis | 254 (93) |
| Cutaneous mycosis | 155 (57) |
| Esophageal/genital mycosis | 153 (56) |
| Onychomycosis | 153 (56) |
| Aphtous stomatitis | 125 (46) |
| Scalp mycosis | 55 (20) |
| Invasive fungal infections | 28 (10) |
| Invasive candidiasis | 10 (4) |
| Other invasive infections | 20 (7) |
| Bacterial infections* | 202 (74) |
| LRI | 129 (47) |
| ENT | 121 (44) |
| Skin | 77 (28) |
| Others† | 24 (9) |
| Mycobacterial infections | 17 (6) |
| Lung disease | 6 (2) |
| Adenitis/skin disease | 5 (2) |
| Disseminated disease | 6 (2) |
| Viral infections* | 103 (38) |
| Cutaneous | 88 (32) |
| Systemic | 23 (8) |
ENT: ear, nose, and throat.
Probable or proven bacterial/viral infection.
Severe acute gastroenteritis, septicemia, bone and joint infections, recurrent urinary tract infections.
Documented pathogens associated with STAT1 GOF mutations
| Associated pathogens . | Documented infections (%) . |
|---|---|
| Mucocutaneous fungal infections | n = 172 |
| Candida albicans | 140 (82) |
| Dermatophytes | 23 (13) |
| Others* | 9 (5) |
| Invasive fungal infections | n = 34 |
| Candida spp. | 10 (29) |
| Pneumocystis jirovecii | 6 (18) |
| Aspergillus spp. | 5 (15) |
| Cryptococcus spp. | 6 (18) |
| Others† | 7 (20) |
| Bacterial infections | n = 99 |
| Staphylococcus aureus | 36 (36) |
| Streptococcus spp. | 20 (20) |
| Pseudomonas aeruginosa | 13 (13) |
| Haemophilus influenzae | 9 (9) |
| Others‡ | 21 (21) |
| Mycobacterial infections | n = 17 |
| M tuberculosis | 6 (35) |
| BCG strain | 5 (30) |
| Others§ | 6 (35) |
| Viral infections|| | n = 162 |
| Herpes simplex | 44 (27) |
| Varicella-zoster | 51 (31) |
| Molluscum contagiosum/warts | 32 (12) |
| CMV or EBV | 25 (15) |
| Others¶ | 10 (6) |
| Parasitic infections# | n = 2 |
| Associated pathogens . | Documented infections (%) . |
|---|---|
| Mucocutaneous fungal infections | n = 172 |
| Candida albicans | 140 (82) |
| Dermatophytes | 23 (13) |
| Others* | 9 (5) |
| Invasive fungal infections | n = 34 |
| Candida spp. | 10 (29) |
| Pneumocystis jirovecii | 6 (18) |
| Aspergillus spp. | 5 (15) |
| Cryptococcus spp. | 6 (18) |
| Others† | 7 (20) |
| Bacterial infections | n = 99 |
| Staphylococcus aureus | 36 (36) |
| Streptococcus spp. | 20 (20) |
| Pseudomonas aeruginosa | 13 (13) |
| Haemophilus influenzae | 9 (9) |
| Others‡ | 21 (21) |
| Mycobacterial infections | n = 17 |
| M tuberculosis | 6 (35) |
| BCG strain | 5 (30) |
| Others§ | 6 (35) |
| Viral infections|| | n = 162 |
| Herpes simplex | 44 (27) |
| Varicella-zoster | 51 (31) |
| Molluscum contagiosum/warts | 32 (12) |
| CMV or EBV | 25 (15) |
| Others¶ | 10 (6) |
| Parasitic infections# | n = 2 |
C glabrata, C tropicalis, C kefir, C membranifaciens, C famata, C dubliniensis, and Malassezia furfur.
Histoplasma, Trichosporon, Coccodioides, Apophysomyces.
Enterobacteriaceae, Campylobacter spp., Stenotrophomonas maltophilia, anaerobic pathogens, Neisseria meningitidis.
M avium, M fortuitum, M mucogenicum, M genavense, or not identified.
Clinically probable or microbiologically confirmed.
HHV6, adenovirus, parvovirus, BK virus, hepatitis C virus, live virus vaccine disease.
Giardiasis, visceral leishmaniasis.
Photographs of patients with STAT1 GOF mutations. (A) Thrush. (B-C) Onycomycosis. (D-F) Cutaneous candidiasis. (G) Dermatophytes. (H) Cutaneous and genital candidiasis. (I) Cutaneous candidiasis. (J) Angular stomatitis. (K) Esophageal candidiasis. (L) Bronchiectasis. (M) Cerebral aneurysm. Complete phenotype of each patient is described in the supplemental Tables 1-3.
Photographs of patients with STAT1 GOF mutations. (A) Thrush. (B-C) Onycomycosis. (D-F) Cutaneous candidiasis. (G) Dermatophytes. (H) Cutaneous and genital candidiasis. (I) Cutaneous candidiasis. (J) Angular stomatitis. (K) Esophageal candidiasis. (L) Bronchiectasis. (M) Cerebral aneurysm. Complete phenotype of each patient is described in the supplemental Tables 1-3.
Bacterial infections
Bacterial infections were observed in 202 of the 274 patients studied (74%; Table 2; supplemental Table 2). LRIs were reported in 129 patients (47%, Table 1) with often recurrent lobar pneumonia, bronchitis, and/or interstitial pneumonia. Streptococcus pneumoniae (n = 13), Pseudomonas aeruginosa (n = 13), Haemophilus influenzae (n = 9), and S aureus (n = 7) were the most frequent pathogens (Table 3). ENT infections were observed in 121 of the 274 patients (44%), mostly as recurrent or chronic sinusitis or otitis media. Recurrent conjunctivitis/keratitis were observed in 39 patients, and 15 patients had blepharitis or ectropion. Recurrent skin infections were found in 77 patients (28%) (Table 2). Folliculitis was the main skin infection, followed by cellulitis, abscesses, and paronychia. The causative agent was documented in 25% of cutaneous infections, with S aureus isolated in most cases. Other probable bacterial infections included recurrent pyelonephritis (n = 8), severe gastroenteritis (n = 8), sepsis (n = 7), or bone and joint infections (n = 3). Seventeen patients (6%) developed mycobacterial diseases. Lung infections were because of M tuberculosis (n = 3) or nontuberculous, environmental mycobacteria (n = 3). Skin disease and adenitis were caused by Bacille Calmette-Guérin (BCG) vaccine or environmental mycobacteria (n = 5), and disseminated disease by BCG (n = 2), M tuberculosis (n=3), or M genavense (n = 1) (Table 3). Two thirds of the patients were presumed to be vaccinated with BCG during childhood according to the global BCG vaccination policies.61
Viral infections
One hundred three patients (of 274, 38%) developed at least 1 systemic or atypical viral infection, or recurrent mucocutaneous viral infections (Table 2; supplemental Table 2). Recurrent mucocutaneous viral infections (rash, stomatitis, vulvitis or keratitis) affected 88 patients (32%). The main causal agents were herpes simplex virus and varicella-zoster virus (VZV) (Table 3). Eighteen patients (7%) had severe chickenpox (ie, rash associated with VZV pneumonitis or cutaneous bacterial superinfection), and 33 (12%) had a history of shingles during childhood that was recurrent in 58% of cases. Thirty-two (12%) patients had recurrent molluscum contagiosum or warts. Severe systemic infections were reported in 23 (8%) of the 274 patients: cytomegalovirus (CMV) and Epstein-Barr virus (EBV) were the viruses most frequently found (Table 3). Uncontrolled CMV viremia requiring antiviral treatment was observed for 8 patients. Six patients had CMV disease with proven organ infection, including 1 patient with retinitis, 2 with ulcerative digestive infections, and 3 with lung infections, one of whom also had encephalitis. One patient had a combined adenovirus and EBV disseminated infection (blood, lung and gut) requiring rituximab with partial efficacy (P234). Chronic active EBV infections (n = 10) were less severe and did not require specific treatment. A few cases of severe human herpesvirus 6 (HHV6) and parvovirus (n = 4) infection were described, resulting in severe sepsis or hemophagocytosis, and 1 patient developed BK virus urinary tract infection with functional consequences. Two patients displayed severe disease because of live virus vaccines (smallpox and measles). Two patients had chronic hepatitis C infection leading to cirrhosis.
Autoimmune and inflammatory diseases
Clinical autoimmunity and/or autoimmune antibodies were documented in 118 (43%) of the 274 patients (Table 4; supplemental Table 3). Median age was 24 years in this subpopulation (range: 3.5-71 years) at the time of the study, and the M/F sex ratio was 0.79. One hundred one (37%) displayed clinical autoimmune manifestations, which represents a much higher rate than the standard population (∼3%),62 and 19% of these patients had >1 clinical autoimmune disorder. Most of these features were related to thyroid disease: hypothyroidism (n = 60) requiring hormone substitution, and hyperthyroidism in 1 patient; 62% of these patients were women. Some patients displayed other autoimmune endocrine diseases, mainly type 1 diabetes mellitus (n = 11). Cutaneous diseases were observed in 28 patients displaying vitiligo, alopecia, or psoriasis. Five female patients displayed SLE with systemic features (ie, vasculitis, serositis or antiphospholipid syndrome with a history of thrombo-embolism), and 1 patient had scleroderma. A few patients displayed pernicious anemia (n = 1) or celiac disease (n = 4) with specific autoantibodies (auto-Abs). Autoimmune hepatitis began before adulthood in 6 patients (67% being men). Hematological autoimmunity (n = 11, 73% of men) consisted of chronic hemolytic anemia or autoimmune thrombocytopenia during childhood. Two patients had ankylosing spondylitis, and 1 patient had multiple sclerosis. Six patients displayed inflammatory bowel disease including Crohn’s disease (n = 2), enteropathy with lymphocytic infiltration (n = 2), and ulcerative colitis (n = 2). Auto-Abs were found in 66 of the 157 patients tested (42%). Most patients with clinical autoimmunity tested were found positive for autoantibodies (n = 49/75, 65%), mostly for anti-nuclear Abs (n = 18), anti-thyroid Abs (n = 21), Abs against other endocrine glands (n = 7), and gastrointestinal Abs (n = 8). Auto-Abs were also detected in the absence of clinical symptoms in 20% of the patients tested (n = 17/84), mostly anti-nuclear Abs (76%) and anti-thyroid Abs (18%).
Other clinical features and outcome of patients with STAT1 GOF mutations
| Noninfectious phenotypes . | Patients (%) . |
|---|---|
| n = 274 . | |
| Autoimmunity/inflammatory disease | 101 (37) |
| Thyroid disease | 61 (22) |
| Other endocrine disease* | 12 (4) |
| Skin disease† | 28 (10) |
| Gastrointestinal disease‡ | 11 (4) |
| Autoimmune hepatitis | 6 (2) |
| Autoimmune cytopenia§ | 11 (4) |
| Others|| | 3 (1) |
| Aneurysm | 17 (6) |
| Cerebral | 14 (5) |
| Extracerebral | 3 (1) |
| Tumor | 17 (6) |
| Benign | 2 (0.7) |
| Squamous cell carcinoma | 11 (4) |
| Gastrointestinal carcinoma | 2 (0.7) |
| Others¶ | 3 (1) |
| Other clinical features | |
| Asthma/eczema | 54 (20) |
| Bone fragility | 5 (2) |
| Clinical outcome | |
| Failure to thrive | 33 (12) |
| Dysphagia/esophageal stenosis | 31 (11) |
| Bronchiectasis | 57 (21) |
| Death | 34 (12) |
| Noninfectious phenotypes . | Patients (%) . |
|---|---|
| n = 274 . | |
| Autoimmunity/inflammatory disease | 101 (37) |
| Thyroid disease | 61 (22) |
| Other endocrine disease* | 12 (4) |
| Skin disease† | 28 (10) |
| Gastrointestinal disease‡ | 11 (4) |
| Autoimmune hepatitis | 6 (2) |
| Autoimmune cytopenia§ | 11 (4) |
| Others|| | 3 (1) |
| Aneurysm | 17 (6) |
| Cerebral | 14 (5) |
| Extracerebral | 3 (1) |
| Tumor | 17 (6) |
| Benign | 2 (0.7) |
| Squamous cell carcinoma | 11 (4) |
| Gastrointestinal carcinoma | 2 (0.7) |
| Others¶ | 3 (1) |
| Other clinical features | |
| Asthma/eczema | 54 (20) |
| Bone fragility | 5 (2) |
| Clinical outcome | |
| Failure to thrive | 33 (12) |
| Dysphagia/esophageal stenosis | 31 (11) |
| Bronchiectasis | 57 (21) |
| Death | 34 (12) |
Diabetes mellitus, Addison’s disease, growth hormone deficiency.
Systemic lupus erythematosus (SLE), vitiligo, psoriasis, alopecia, scleroderma.
Biermer anemia, celiac disease, colitis.
Immunological anemia or thrombocytopenia.
Multiple sclerosis, ankylosing spondylitis.
Melanoma, basal cell carcinoma, acute lymphoblastic leukemia.
Other clinical features
Aneurysms (n = 17/274, 6%; Table 4; supplemental Table 3; Figure 2) occurred at a higher rate than the standard population (∼3%)63 and were diagnosed at a median age of 23 years (range: 3-50 years), which is much younger than the standard population (∼50 years).63 There was no difference between the sexes. Aneurysms tend to be more frequent in patients with underlying autommunity (P = .05), type I diabetes in particular (27% vs 5%, P <. 01). Diagnosis was based on symptoms and radiologically confirmed in 15 patients. Systematic radiological investigations identified 2 additional patients. Cerebral imaging was also carried out for 25 other asymptomatic patients and yielded normal results. Fifty percent of symptomatic patients endured hemorrhages; the others had abdominal pain, and neurological manifestations, such as hemiplegia, seizures or attention lapses. Most aneurysms were located in the cerebral vascular system (82%). Most affected patients had multiple aneurysms in the CNS, mostly in the basilar trunk, vertebral arteries, and cerebral and intracranial carotid arteries. Extracerebral aneurysms were found in the abdominal aorta, iliac arteries, and lung arteries (n = 3). Histological and microbiological investigations were performed in 1 patient (P27, supplemental Table 3) and were negative.44 The other neurological features observed were cerebral vasculitis (n = 3), epilepsy (n = 3), polyneuropathy (n = 1), multiple sclerosis (n = 1), and hemiplegia of unknown origin (n = 1). Cognitive disability was diagnosed in 8 patients during childhood, a frequency slightly higher than that in the general population.64 Fourteen patients had cutaneous, gastrointestinal, or laryngeal carcinoma, 11 of which were squamous cell carcinomas, 1 patient had melanoma, and another one acute leukemia, leading to an overall cancer rate of 5.8% (95% confidence interval 3.03% to 8.57%) (Table 4). The M/F ratio was 1.0. We compared this cancer rate with that observed in the reference 2000 US Standard population.65,66 To account for the low median age of the STAT1 GOF patients, we computed the expected cancer rate in the reference population as a weighted average of the age-specific cancer rates, in which the weights were the proportions of STAT1 GOF patients in the corresponding age groups. The expected age-adjusted cancer rate in the reference population was found to be 1.1%, below the lower bound of the confidence interval of the rate in the population of patients studied here. This result supports that STAT1 GOF patients may have an increased risk of cancer. Carcinoma were more frequent in patients with a history of esophagitis (10% vs 2%, P = .002). Two patients had benign tumors of vascular origin. Finally, other diseases, such as asthma, eczema, and signs of allergy were observed in 54 patients (20%), a frequency similar to that found in the general population and without unusual severity.67
Preventive and curative treatment
Two hundred two (of 274, 74%) patients needed long-term antifungal treatment, mostly systemic (Table 5). Fluconazole was the major agent used for first-line treatment, followed by itraconazole or posaconazole. Clinical resistance to at least 1 antifungal was observed in 78 patients of the 202 patients treated with long-term antifungal therapy (39%), and in 8 of the 53 patients treated intermittently (15%). Twenty-five treated patients displayed resistances to >1 antifungal (10%). Results for strain susceptibility were available for 40% of the patients with CMC resistant to treatment and revealed a high level of resistance to azoles (98%). Most of these patients required second- or third-line treatments, such as voriconazole, echinocandins, terbinafine or liposomal amphotericin B. Patients with clinical resistance to antifungals had globally a more severe phenotype (recurrent pneumonia: 51% vs 25% P < .001, systemic fungal infections: 17% vs 7% P = .01, mortality: 21% vs 7%, P = .001). Antibacterial prophylaxis was administered to 66 patients (of 274, 24%), for recurrent LRI or cutaneous staphylococcal disease; co-trimoxazole was the main agent used (Table 5). Thirty percent of patients with recurrent pneumonia received polyvalent IV IgG infusions (14% of all patients). One patient received GM-CSF infusions, IFN-α, and then acitretin, with no major improvement of CMC or herpes virus infections. Five patients received G-CSF, leading to a marked improvement in CMC lesions in 1 patient only. One patient received IFN-γ from 6 to 15 years, with no detectable effect on CMC or LRIs. One patient (P116) was treated with JAK inhibitor ruxolitinib that led to significant clinical improvement of CMC without complete clearance of the disease.58 Allogeneic hematopoietic stem cell transplantation (HSCT) was performed in 5 patients with severe CMC and recurrent bacterial or systemic viral infections; 3 of them died several months after HSCT, 1 from persistent hemophagocytic lymphohistiocytosis despite 3 HSCT (P157, phenotypically HLA-identical, cord blood transplantation, and haploidentical HSCT), 1 from disseminated CMV at 30 years (P160, fully matched HSCT), and the other from interstitial lung disease at 2 years (P190, HLA-identical sibling HSCT). The other 2 are currently alive without serious complications (P161, cord blood transplantation from mismatched donor and P234, phenotypically HLA-identical HSCT). Six patients underwent immunosuppressive treatment of severe autoimmune disorders, and had a good clinical response, without serious infectious complications. One patient underwent radiotherapy for squamous cell carcinoma and recovered fully, and 2 others had rituximab for uncontrolled systemic EBV infection.
Treatments of patients with STAT1 GOF mutations
| Treatment . | Patients (%) . |
|---|---|
| n = 274 . | |
| No antifungal treatment | 19 (7) |
| Intermittent antifungal treatment | 53 (19) |
| Current long-term antifungal treatment | 202 (74) |
| Local treatment only | 8 (3) |
| Oral fluconazole | 150 (55) |
| Oral posaconazole/itraconazole | 53 (19) |
| Oral voriconazole | 19 (7) |
| IV echinocandins | 6 (2) |
| Oral terbinafine | 3 (1) |
| IV amphotericin B | 7 (3) |
| Antibiotic prophylaxis | 66 (24) |
| Co-trimoxazole | 41 (15) |
| Macrolides | 20 (7) |
| Others* | 12 (4) |
| Antiviral prophylaxis | 4 (1) |
| Polyvalent immunoglobulins | 37 (14) |
| Immunotherapy† | 8 (3) |
| Immunosuppressive therapies‡ | 6 (2) |
| Hematopoietic stem cell transplantation (HSCT) | 5 (2) |
| Treatment . | Patients (%) . |
|---|---|
| n = 274 . | |
| No antifungal treatment | 19 (7) |
| Intermittent antifungal treatment | 53 (19) |
| Current long-term antifungal treatment | 202 (74) |
| Local treatment only | 8 (3) |
| Oral fluconazole | 150 (55) |
| Oral posaconazole/itraconazole | 53 (19) |
| Oral voriconazole | 19 (7) |
| IV echinocandins | 6 (2) |
| Oral terbinafine | 3 (1) |
| IV amphotericin B | 7 (3) |
| Antibiotic prophylaxis | 66 (24) |
| Co-trimoxazole | 41 (15) |
| Macrolides | 20 (7) |
| Others* | 12 (4) |
| Antiviral prophylaxis | 4 (1) |
| Polyvalent immunoglobulins | 37 (14) |
| Immunotherapy† | 8 (3) |
| Immunosuppressive therapies‡ | 6 (2) |
| Hematopoietic stem cell transplantation (HSCT) | 5 (2) |
Nebulized colimycin, topical fucidic acid, fluoroquinolone, tetracycline, amoxicillin.
Granulocyte–colony stimulating factor (G-CSF)/granulocyte macrophage–colony stimulating factor (GM-CSF), interferon (IFN)α/γ, rituximab.
Cyclosporine, aziathoprine, corticoids, or mycophenolate mofetil.
Clinical outcome
Thirty-three patients (of 274, 12%) failed to thrive (Table 4). Thirty-one patients (11%) developed secondary gastrointestinal complications, such as dysphagia (n = 19) or esophageal stenosis (n = 12). Most of them had a history of recurrent esophageal candidiasis (n = 30, 97%). Bronchiectasis and cystic pulmonary lesions developed in 57 patients (of 274, 21%) (Table 4; Figure 2), all with a previous history of recurrent pneumonia or bronchitis, and displaying acute secondary infectious episodes caused by P aeruginosa (n = 11 out of 57, 19%), S aureus (n = 6, 11%), nontuberculous mycobacteria (n = 4, 7%) or Enterobacteriaceae (n = 2, 3%). One of these patients also had an associated pneumatocyst, and 3 other patients underwent lobectomy. Thirty-four patients (of 274, 12%) died (Table 4; supplemental Table 3) at a median age of 30 years (range: 1-58 years). The main causes of death were severe infections (n = 13, 38%), ie, disseminated BCG disease, histoplasmosis, coccidioidomycosis, CMV, S aureus septicemia or bacterial LRI, at a median age of 17 years (range: 1-52 years), cancer (n = 8, 24%), at a median age of 43 years (range: 33-58 years), and cerebral hemorrhage because of aneurysm (n = 5, 15%), at a median age of 9 years (range: 4-34 years). Overall, cumulative survival rate at 60 years of age was significantly lower (31%) in patients who developed invasive infection, cancer, and/or symptomatic aneurysm than those without (87%) any of these 3 complications (Figure 1B). Other causes of death possibly associated with STAT1 GOF phenotypes included fulminant hepatitis (1 patient from an unknown cause, and 1 from autoimmune hepatitis), and complication of HSCT in 3 patients. Three patients died of unrelated causes, at the ages of 10, 46, and 50 years. Lymphocyte cell subset abnormality (low CD4+ and/or CD19+ lymphocyte counts) was associated with a higher mortality rate (19% vs 6%, (P = .004), and 25% vs 7%, (P < . 001), respectively).
Discussion
We show here that CMC is the most common infectious manifestation in patients carrying STAT1 GOF mutations, and is frequently resistant to azole treatments. Surprisingly, these patients often display viral, bacterial, and other fungal infections. In addition, these patients also develop various types of autoimmunity, as well as aneurysms and carcinomas, much more often than the general population. The presence of these latter complications accounts for a poor outcome. The penetrance of CMC is very high, as only 6 (2%) STAT1 GOF patients (2.8% excluding all probands) never had CMC at a median age of 32 years. CMC typically begins in early childhood, although it may first present up to the third decade of life, and signs and symptoms varied within and between families.20 In these patients, CMC is most likely the consequence of impaired IL-17A and IL-17F immunity,19-21,23,39,42,44 as it is also seen in patients with inborn errors of IL-17A/F immunity.16-18 Circulating IL-17A-producing T cells counts are low in most but not all patients tested (82%). Normal counts therefore cannot exclude a diagnosis of a STAT1 GOF mutation. The frequency of CMC in the cohort described here may be overestimated as a result of an ascertainment bias. Clearly, STAT1 GOF mutations underlie biological phenomena that can cause markedly different phenotypes that are rarely mutually exclusive, as evidenced by CMC-free patients. Other fungal infections included mostly superficial dermatophytosis, without the deep dermatophytosis seen in some CARD9-deficient patients.14,68 Invasive infections by a variety of yeasts, molds, and dimorphic fungi were observed in some patients.23,34,37,40 Cutaneous and bronchopulmonary infections caused by S aureus were also observed, as in patients with AD-HIES. The occurrence of staphylococcal skin disease in some patients with CMCD and inborn errors of IL-17 immunity1,16,18 suggests the involvement of 1 or more IL-17 cytokines. Its occurrence in patients with auto-Abs against IL-6 suggests that impaired STAT3-dependent IL-6 signaling may also be involved.20,69,70 Respiratory tract infections may be favored by low IgA, IgG2, and/or IgG4 levels as well as a poor Ab response, as documented in some patients.71,72 The occurrence of mycobacterial and viral infections is paradoxical, as biallelic LOF STAT1 mutations underlie such infections by impairing IFN-γ and IFN-α/β responses, respectively, while GOF STAT1 mono-allelic mutations enhance signaling downstream of these cytokines.20,23,37,45,73 Viral disease might be because of exhaustion of virus-specific T cells, as described for patients with AD HIES,74 and mycobacterial disease to refractory responses to IFN-γ.37
Patients with GOF STAT1 mutations also displayed autoimmunity, aneurysms, and malignancies. Autoimmunity is not observed in patients with inborn errors of IL-17A/F immunity or in patients with AD HIES. Other monogenic autoimmune disorders may shed more light on the pathogenesis of autoimmunity in patients with STAT1 GOF mutations.75 IPEX syndrome, caused by FOXP3 deficiency, underlies forms of autoimmunity that occasionally overlap with those in STAT1 GOF patients.23,75 STAT1 GOF mutations however do not seem to affect FOXP3 expression and the development of Tregs.23 The autoimmune features observed in patients with STAT1 GOF mutations also overlap with those of APS-1 patients (with mutations in AIRE). In addition to CMC, APS-1 patients usually present hypoparathyroidism, adrenal insufficiency, and, occasionally, thyroiditis, autoimmune enteropathy, vitiligo, alopecia, diabetes, and hepatitis.3,76 Although the corresponding patients share autoimmune phenotypes, LOF AIRE and GOF STAT1 mutations have not been mechanistically connected yet. The enhanced autoimmunity of patients with STAT1 GOF mutations is likely to result from stronger IFN-α/β signaling, as some of these autoimmune features are observed in patients treated with recombinant IFN-α (eg, thyroiditis) and in patients with type I interferonopathies (eg, SLE).77,78 It remains unclear why the various inborn errors resulting in enhanced IFN-α/β immunity give rise to such diverse and only partially overlapping phenotypes.77,79
The outcome of patients with STAT1 GOF mutations is poor. Premature death (12%) results from infections (38%), aneurysms (15%), cancers (24%), and autoimmune hepatitis (6%). Infections remain a major cause of premature death in these patients, with a high proportion of severe and/or recurrent LRI infections, leading to lung sequelae, viral, and invasive fungal infections.1,2 The proportion of patients with cerebral aneurysm has probably been underestimated, as radiological investigations were performed for only 42 patients, including 15 patients with clinical signs suggestive of aneurysm. Given the high morbidity and mortality of cerebral aneurysms, systematic radiological screening is probably warranted in all patients, when the diagnosis is made, and should be repeated during life. Aneurysms may form because of conjunctive tissue abnormalities, as in patients with STAT3 deficiency,80 and/or impaired IL-17 immunity, as shown in animal models.81 Their pathogenesis may also be autoimmune, as in cerebral vasculitis, or infectious, as in systemic Candida infection.1 Patients with aneurysms did not differ markedly from the others in terms of invasive fungal infection rates (P = .6), but they seemed to display more autoimmune disorders. Patients with an interferonopathy caused by SAMHD1 mutations also display cerebral vasculitis and aneurysm.77,82 The frequency of skin and ENT carcinomas was high, and probably better estimated than that of cerebral aneurysm. These conditions are better understood and probably result, at least in part, from CMC and the chronic mucocutaneous inflammation (especially esophagitis) it causes.83 Enhanced IFN production is unlikely to play a direct role, as these cytokines have antitumoral activity.84 Patients with esophagitis and dysphagia should be regularly screened for tumor by sequential biopsies of the esophagus. Taken together, STAT1 GOF-associated AD CMCD should not be considered benign and should be handled at centers with experience in the diagnosis and management of such patients.
The heterogeneity of clinical care for the patients in this cohort makes it difficult to issue uniform recommendations concerning optimal management. The high rate of CMC resistance to antifungal treatments is a major issue. Indeed, azole resistance necessitates the intravenous use of alternative antifungals (amphotericin B, echinocandins) that could lead to toxicity and major lifestyle changes. Furthermore, as resistance to treatment is associated with severe infectious phenotype (systemic bacterial or fungal infections) and a poor outcome, these patients should be seen more frequently. However, long-term antifungal therapy remains the first line for CMC treatment, whereas antibiotic prophylaxis and IgG infusion should be considered for patients with recurrent LRIs, with or without detectable Ab deficiency. Second line therapies, such as GM-CSF and G-CSF treatments have been proposed as a way of enhancing IL-17 T-cell differentiation.85-87 However, despite an encouraging recent report,41 these adjuvant therapies were useful in only 1 of the 5 patients in which they were tried. Treatments targeting the JAK-STAT pathway, such as the JAK1/2 inhibitor ruxolitinib, which has been approved for myelofibrosis treatment, have shown significant clinical efficiency and might become the treatment of choice for severe CMC resistant to antifungals.30,58 HSCT was performed in 5 patients with severe and recurrent fungal and viral infections,25 but 3 of them died. HSCT does not appear to be a viable option at the present time. However, pilot studies with closely matched donors should be considered. Other potential immunotherapies such as recombinant IL-17A or IL-17F or inhibitors of STAT1 activity for specific use in patients with GOF STAT1 mutations may prove more useful. IFN-α/β-blocking antibodies might also alleviate autoimmune features and may be considered in the future. The options must be weighed up carefully, bearing in mind the various anti-infectious, antitumor, and autoimmune effects of each cytokine or Ab. In conclusion, STAT1 GOF mutations are the most common known genetic etiology of CMCD and are found in about half the patients studied.1,88 However, given the unexpected broad array of clinical diseases revealed by our current study, physicians should also evoke STAT1 GOF in patients whose candidiasis is a minor, incidental finding, and even in patients without candidiasis. Disease severity results from the deleterious impact of CMCD on quality of life, and/or the poor outcome associated with infections, autoimmunity, aneurysm, and carcinoma.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their relatives, as well as Lahouari Amar and Yelena Nemirovskaya.
The Laboratory of Human Genetics of Infectious Diseases was supported by the French National Research Agency (ANR) under the “Investments for the future” program (ANR-10-IAHU-01), GENCMCD grant (ANR-11-BSV3–005-01), HGDIFD (ANR-14-CE15-0006), and Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (# ANR-10-LABX-62-IBEID); INSERM; University Paris Descartes; the Jeffrey Modell Foundation–Translational Research Program; the Jeffrey Modell Centers Network; the Rockefeller University; the St. Giles Foundation; and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant U01AI109697). This work was also supported by the ERA-Net for Research Programmes on Rare Diseases “E-RARE EURO CMC” (ANR-14-RARE-0005-02). B.G. was supported by the Helmholtz Center grant DZIF (8000805-3_TTU_IICH 07.801). J.R. was supported by Gebert Rüf Stiftung–programme Rare Diseases–New Approaches, EUFP7 CELL-PID, EUFP7 NET4CGD, ZIHP. C.R.-G. was supported by the Ministerio de Sanidad, Spain (grant PI13/1456), from the Regional Development Fund–European Social Fund (FEDER-FSE).
Authorship
Contribution: J.T. and A.P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; J.T., J.-L.C., and A.P. did the scientific literature search and were responsible for the study design; all authors participated in the patients’ inclusion and data collection; J.T. and A.P. centralized the data; J.T. undertook the data analysis; all authors interpreted the data; J.T. and A.P. created the figures; J.T., J.-L.C., and A.P. prepared the first draft of the manuscript; and all authors were involved in the writing and/or revision of the manuscript.
Conflict-of-interest: The authors declare no competing financial interests.
A complete list of the members of the International STAT1-GOF Study Group appears in “Appendix.”
Correspondence: Anne Puel, Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM UMR1163, Imagine Institute, Necker-Enfants Malades Hospital, 24 Boulevard du Montparnasse, 75015 Paris, France; e-mail: anne.puel@inserm.fr.
Appendix: study group members
The members of the International STAT1-GOF Study Group: Sophie Cypowyj, Caroline Thumerelle, Antoine Toulon, Jacinta Bustamante, Natalia Tahuil, Aicha Salhi, Sorina Boiu, Charu Chopra, Daniela Di Giovanni, Liliana Bezrodnik, Jeannette Boutros, Caroline Thomas, Gina Lacuesta, Sarah Jannier, Anne-Sophie Korganow, Catherine Paillard, David Boutboul, Mélanie Bué, Aude Marie-Cardine, Sophie Bayart, Mélanie Migaud, Laurence Weiss, Marina Karmochkine, Juan-Miguel Garcia-Martinez, Jean-Louis Stephan, Philippe Bensaid, Guy-Patrick Jeannoel, Torsten Witte, Ulrich Baumann, Thomas Harrer, Carmen Navarrete, Antony Terance Benjamin, Davide Firinu, Claudio Pignata, Paolo Picco, David Mendoza, Saul Oswaldo Lugo Reyes, Carlos Torres Lozano, Margarita Ortega-Cisneros, Mariana Cortina, Mehrnaz Mesdaghi, Mohammad Nabavi, Teresa Español, Maía Teresa Martínez-Saavedra, Nima Rezaei, Samaneh Zoghi, Malgorzata Pac, Vincent Barlogis, Gabriel Revon-Rivière, Yishai Haimi-Cohen, Ronen Spiegel, Dan Miron, Jabir Bouchaib, Lizbeth Blancas-Galicia, Beata Toth, Barbara Drexel, Pierre Simon Rohrlich, Olivier Lesens, Miriam Hoernes, Elizabeth Drewe, Mario Abinum, Julie Sawalle-Belohradsky, Gerhard Kindle, Mark Depner, Lili Milani, Tiit Nikopensius, Maido Remm, Ulvi Gerst Talas, Mark Tucker, Mary Willis, Stephanie Leonard, Hilaire Meuwissen, Ronald M. Ferdman, Mark Wallace, Mukesh M. Desai, Prasad Taur, Raffaele Badolato, Beata Soltesz, Christina Schnopp, Annette F. Jansson, Deniz Ayvaz, Nadejda Shabashova, Liudmyla Chernyshova, Anastasia Bondarenko, Despina Moshous, Benedicte Neven, Chahinez Boubidi, Fatima Ailal, Giuliana Giardino, Stefano Del Giacco, Marie-Elisabeth Bougnoux, Kohsuke Imai, Teppei Okawa, Yoko Mizoguchi, Yusuke Ozaki, Masato Takeuchi, Akira Hayakawa, Birgit Lögering, Kristian Reich, Timo Buhl, Kilian Eyerich, Martin Schaller, Peter D. Arkwright, Andrew R. Gennery, Andrew J. Cant, Adilia Warris, Stefanie Henriet, Najla Mekki, Ridha Barbouche, Imen Ben Mustapha, Christine Bodemer, Michel Polak, Emmanuel Grimprel, Pierre-Régis Burgel, Alain Fischer, Olivier Hermine, Marianne Debré, Dilara Kocacyk, Fatima Dhalla, Smita Y. Patel, Leen Moens, Filomeen Haerynck, Melissa Dullaers, Levi Hoste, Ozden Sanal, Sara Sebnem Kilic, Joachim Roesler, Fanny Lanternier, Olivier Lortholary, Claire Fieschi, Joseph A. Church, Chaim Roifman, Araya Yuenyongviwat, Pärt Peterson, Stéphanie Boisson-Dupuis, Laurent Abel, Beatriz E. Marciano, and Mihai G. Netea.
References
Author notes
J.H., M.O., M.L.G., J.C.A.B., M.O.-C., and F.F. contributed equally to this study.
K.M.G., A.E., J.V.M., Y.C., L.A.K., B.B., S.B., A.B., C.R.-G., I.M., K.K., and J.R. contributed equally to this study.
E.D.R., S.R., B.G., F.L.v.d.V., C.T.-H., C.P., L.M., T.M., M.K., D.L., J.D.M., and S.H. contributed equally to this study.
J.-L.C. and A.P. contributed equally to this study.