To the editor:
Chronic lymphocytic leukemia (CLL) is a B-cell lymphoproliferative disorder partly dependent on the B-cell receptor (BCR) signaling pathway. The novel BCR signal transduction inhibitor ibrutinib has emerged as an effective therapeutic agent for CLL.1 Ibrutinib covalently binds to and inhibits Bruton tyrosine kinase (BTK), a critical component of BCR signaling thought to be integral to B-cell development, maturation, differentiation, and migration. Ibrutinib is currently approved for the treatment of patients with relapsed CLL or CLL with del(17p), relapsed mantle cell lymphoma, and Waldenström macroglobulinemia.2-6 Major toxicities of ibrutinib include bleeding, fatigue, arthralgia, infection, and atrial fibrillation.7,8 One prior case of ibrutinib-associated pneumonitis has been reported.9 Herein, we report 4 cases of relapsed/refractory CLL patients who developed pneumonitis.
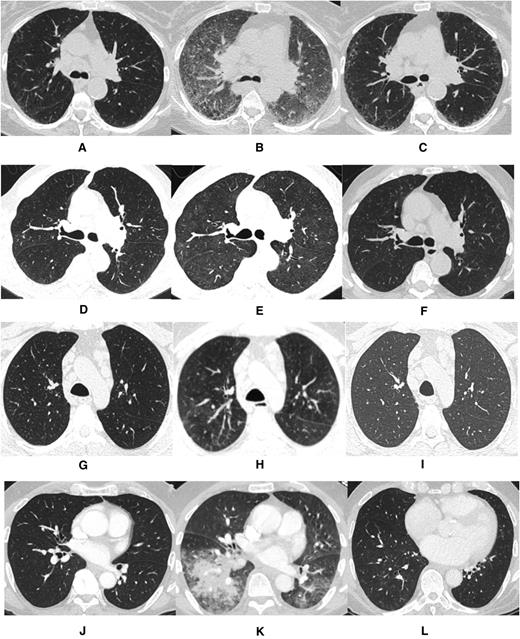
The first case describes a 71-year-old woman with del(17p) del(11q)-positive relapsed CLL who was treated with ibrutinib at 420 mg daily 9 years after initial diagnosis. Prior therapy included fludarabine and rituximab. One month following ibrutinib initiation, she was hospitalized with dyspnea and hypoxia. Infectious workup was negative. A computerized tomography (CT) scan of the chest revealed widespread interstitial ground glass opacities not noted on CT imaging obtained 4 weeks prior to ibrutinib exposure (Figure 1A-B). Transbronchial biopsy and cytology revealed fragments of alveolated lung parenchyma with chronic interstitial inflammation and organization associated with loosely formed nonnecrotizing granulomas without evidence of viral infection or malignancy (Figure 2). Prednisone, at 40 mg daily, was initiated. The patient improved within 1 week, and ibrutinib was resumed at 420 mg daily on discharge. Three months following discharge, a CT scan of the chest demonstrated resolution of ground glass opacities (Figure 1C). Ten months following her initial hospitalization, the patient presented with dyspnea and increased sputum production without fever. An infectious workup was negative. A CT scan of the chest revealed centrilobular emphysema and superimposed peripheral reticular interstitial lung disease consistent with pulmonary fibrosis. Ibrutinib was held, and prednisone, 30 mg daily, was initiated. The patient improved and was discharged on steroids; ibrutinib was permanently discontinued.
Computed tomography imaging for each case prior to ibrutinib exposure, during pneumonitis while on ibrutinib, and following ibrutinib discontinuation. (A,D,G,J) Pre-ibrutinib; (B,E,H,K) during ibrutinib; (C,F,I,L) post-ibrutinib.
Computed tomography imaging for each case prior to ibrutinib exposure, during pneumonitis while on ibrutinib, and following ibrutinib discontinuation. (A,D,G,J) Pre-ibrutinib; (B,E,H,K) during ibrutinib; (C,F,I,L) post-ibrutinib.
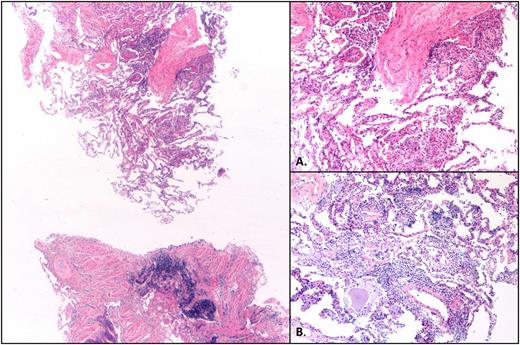
Hematoxylin and eosin–stained sections reveal patchy lung parenchymal involvement by intra-alveolar loose fibrous plugs, intra-alveolar fibrin, reactive pneumocytes, and mild chronic interstitial inflammation (×2.5). (A) Higher magnification (×10) of loose fibrous plugs in alveolar spaces and indistinct granuloma. (B) Higher magnification (×10) of focus with chronic interstitial inflammation.
Hematoxylin and eosin–stained sections reveal patchy lung parenchymal involvement by intra-alveolar loose fibrous plugs, intra-alveolar fibrin, reactive pneumocytes, and mild chronic interstitial inflammation (×2.5). (A) Higher magnification (×10) of loose fibrous plugs in alveolar spaces and indistinct granuloma. (B) Higher magnification (×10) of focus with chronic interstitial inflammation.
The second case involves a 73-year-old man with del(11q)-positive relapsed CLL who was treated with ibrutinib at 420 mg daily 15 years after initial diagnosis. Prior CLL therapies included rituximab, fludarabine-cyclophosphamide-rituximab (FCR), rituximab-bendamustine (BR), ofatumumab, and anti-CD19 autologous chimeric antigen receptor T cells. Four months after initiating ibrutinib, the patient was hospitalized for dyspnea. A chest CT scan revealed diffuse ground glass opacities, absent in a CT scan performed before ibrutinib exposure (Figure 1D-E). Patchy areas of consolidation in the left lower lobe suggested stable chronic low-grade aspiration. Transbronchial biopsies revealed bronchial mucosa and alveolated lung parenchyma with reactive lymphoid infiltrate and organizing pneumonia with occasional eosinophils and neutrophils. Cytology revealed acute inflammation without evidence of malignancy or infectious etiology. Ibrutinib was discontinued, and prednisone 60 mg daily was administered for possible pneumonitis secondary to ibrutinib exposure. The patient experienced marked improvement, and ibrutinib was permanently discontinued. A follow-up CT scan of the chest confirmed radiographic resolution (Figure 1F).
The third case describes a 55-year-old man with relapsed del(11q)-positive CLL who was treated with ibrutinib at 420 mg daily 3 years after initial diagnosis. Prior CLL therapies included FCR and BR. Four months after initiating ibrutinib, the patient developed stomatitis and neutropenia requiring dose reduction to ibrutinib 280 mg daily. Subsequently, he was hospitalized for dyspnea, and a high-resolution chest CT scan revealed diffuse ground glass opacities in the lungs with small nodular opacities in the left lower lobe and bilateral trace pleural fluid (Figure 1G-H). Transbronchial biopsies and cytology revealed acute inflammation without evidence of malignancy or infection. Ibrutinib was discontinued, and prednisone initiated at 60 mg daily. The patient’s symptoms resolved, and ibrutinib was permanently discontinued. A follow-up CT scan confirmed radiographic improvement (Figure 1I).
The final case is a 71-year-old man with del(11q) del(13q)-positive relapsed CLL who was treated 10 years after initial diagnosis with ibrutinib at 420 mg daily. Prior CLL therapies included FCR; BR; and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. Three months after initial ibrutinib exposure, the patient developed low-grade fever and cough. A CT scan of the chest revealed bilateral ground glass opacities and new consolidation in the right lower lobe absent on a CT scan obtained prior to ibrutinib (Figure 1J-K). The patient was initially treated with intravenous antibiotics, which were discontinued following a negative infectious workup. Transbronchial biopsies and cytology revealed unremarkable bronchial epithelial cells with macrophages without evidence of neoplasia. Ibrutinib was discontinued, and the patient was treated with prednisone 30 mg daily. The patient’s symptoms and respiratory status improved. A subsequent CT scan showed resolution of the radiographic abnormalities (Figure 1L).
To our knowledge, this is the first case series describing pneumonitis associated with ibrutinib therapy. All 4 patients were successfully treated with corticosteroids, and the previously detected radiographic changes resolved.
Importantly, all patients in this series underwent an extensive evaluation that included pulmonary consultation, transbronchial biopsies, and infectious workup (blood cultures, serum cytomegalovirus polymerase chain reaction, rapid viral respiratory panel). Although prior therapies and the possibility of an occult preexisting pulmonary disease might explain some of the findings, the fact that new radiographic changes were detected after commencement of ibrutinib therapy suggests that they were related to ibrutinib. Importantly, imaging abnormalities correlated with findings obtained from transbronchial biopsy specimens, which can be nonspecific in drug-induced pneumonitis, but consistently revealed patterns of organizing pneumonia, interstitial inflammatory infiltrates, or granulomas that did not stain positive for acid-fast bacilli or fungi.10,11 Further, these findings and the underlying symptoms all resolved after stopping ibrutinib. In 1 case, rechallenging the patient with ibrutinib led to recurrence of these respiratory symptoms.
Similar observations of pulmonary toxicity have been reported with other targeted therapies such as mammalian target of rapamycin inhibitors,12,13 phosphatidylinositol 3-kinase inhibitors,14 and spleen tyrosine kinase (SYK) inhibitors.15 In fact, the incidence of pneumonitis in idelalisib-treated CLL patients is reported at 4%.14 Also, in a phase 2 clinical trial with a combination of the SYK inhibitor GS9973 and idelalisib, 13.6% of CLL and/or non-Hodgkin lymphoma patients developed severe pneumonitis leading to early termination of the study.15 The underlying pathophysiology of how signal transduction inhibitors are associated with pulmonary toxicities remains to be defined.
Given the findings of a nonmalignant inflammatory infiltrate, we hypothesize that inhibiting signal transduction pathways enhances expression of proinflammatory cytokines and the innate immune system. BTK also appears to serve as a critical mediator of lipopolysaccharide-induced dendritic cell maturation and macrophage polarization. Several studies have reported an increase in alveolar infiltration of T helper 2 proinflammatory cytokines in BTK-deficient mice, resulting in airway inflammation.16,17
Our findings have important clinical implications. We propose that patients may receive counseling about this potential toxicity prior to initiating ibrutinib therapy and that any respiratory illness be taken seriously and evaluated effectively. Management strategies could include dose interruption and/or drug discontinuation along with initiation of steroids in severe cases. Further studies and better understanding of this toxicity are needed as the use of ibrutinib for treating lymphoid malignancies is expected to increase in the near future.
Authorship
Contribution: A.R.M. designed the study, collected data, wrote and edited the manuscript, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; P.I., C.D., and L.S. collected data and wrote and edited the manuscript; A.H.K. performed the radiology review; S.B. performed the pathology review; A.G. was involved in drug dosing, management, and the writing and editing of the paper; S.N., D.L.P., J.S., and S.J.S. provided patient care and edited the manuscript; and C.N. wrote and edited the manuscript.
Conflict-of-interest-disclosure: J.S. receives research funding from Seattle Genetics, Celegene, Pharmacyclics, and MedImmune. D.L.P. receives research funding, intellectual property, and royalty payments from Novartis. His spouse is employed by Genentech. S.J.S. receives research funding from Pharmacyclics, Novartis, Gilead, Janssen, Celgene, and Hoffman-LaRoche; receives consultancy fees from Pharmacyclics, Celgene, and Genentech; and serves on the Nordic Nanovector Board of Directors or advisory committees. A.R.M. receives research funding from TG Therapeutics, Acerta, Gilead, Celgene, and Pharmacyclics and has received consulting fees from TG Therapeutics, Gilead, and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Anthony Mato, Perelman Center for Advanced Medicine, 2nd Floor, West Pavilion, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: anthony.mato@uphs.upenn.edu