Abstract
Extramedullary myeloma (EMM) is defined by the presence of plasma cells (PCs) outside the bone marrow in a patient with multiple myeloma (MM). Using sensitive imaging techniques including magnetic resonance imaging and positron emission tomography/computed tomography, EMM may be found in up to 30% of MM patients across the overall disease course. The molecular mechanisms underlying the hematogenous spread of PCs outside the bone marrow are only partially known and involve hypoxia and an altered expression of adhesion molecules. Extramedullary disease is associated with adverse prognostic factors (ie, high lactate dehydrogenase level, 17p deletion, and high-risk gene expression profile). The prognosis of EMM is poor, and the median overall survival of patients who experience an extramedullary relapse is <6 months. The adverse prognosis is less pronounced in patients with bone-related plasmacytomas than in those with hematogenous EMM. EMM patients should be considered as having high-risk myeloma and treated accordingly. However, EMM clinical situations are extraordinarily heterogeneous, and their management is particularly challenging. In the present review, a case-and-comment format is used to describe our approach to the management of EMM.
Introduction
Multiple myeloma (MM) is a mature B-cell neoplasm that accounts for 13% of all hematologic malignancies and has an age-adjusted incidence rate of nearly 6 per 100 000 persons per year.1 MM primarily affects older individuals with a median age at the time of diagnosis of nearly 70 years.2 Over the past decade, the median survival of myeloma patients has almost doubled from 4 to 8 years.3 This remarkable improvement is mostly because of the use of high-dose therapy followed by autologous stem cell transplantation (ASCT), in addition to the widespread incorporation of novel agents including immunomodulatory drugs (IMiDs; thalidomide and lenalidomide) and a proteasome inhibitor (PI; bortezomib). The arsenal of effective novel agents for patients with relapsed disease is constantly increasing and now includes a next-generation IMiD (pomalidomide), next-generation PIs (carfilzomib, ixazomib, oprozomib, and marizomib), a histone deacetylase inhibitor (panobinostat), and monoclonal antibodies (directed against CD38: daratumumab, isatuximab [SAR650984], and MOR202; or against SLAMF7: elotuzumab).4
MM is defined by the presence of ≥10% of clonal plasma cells (PCs) in the bone marrow (or a biopsy-proven extramedullary plasmacytoma) and by the evidence of end-organ damage attributed to the PC disorder (hypercalcemia, renal insufficiency, anemia, and bone lesions).5 For most myeloma patients, the PC proliferation is restricted to the bone marrow. However, a subset of MM patients develops extramedullary myeloma (EMM), defined by the presence of clonal PCs outside the bone marrow.6 The management of EMM is particularly challenging, and important questions relating to its definition, diagnosis, and treatment exist.
The clinical spectrum of EMM
The term “extramedullary myeloma” itself is confusing because in the myeloma literature it may refer to the following different entities, which are summarized in Table 1: (1) Bone-related plasmacytomas are tumor masses affecting the axial skeleton (ribs, vertebrae, skull, sternum, and pelvis), which originate from the underlying bone marrow through the disruption of the cortical bone.6 (2) Extramedullary disease is secondary to a hematogenous spread and refers to soft-tissue tumors arising from, or the PC infiltration of, an anatomical site distant from the bone marrow (mostly liver, skin, CNS, pleural effusion, kidneys, lymph nodes, and pancreas).6,7 Interestingly, extramedullary spread can be triggered by an invasive procedure (surgery or catheter insertion) or by a bone fracture.6,8,9 (3) Plasma cell leukemia (PCL) is an aggressive variant of myeloma defined by the presence of circulating PCs (>20% and/or absolute count >2 × 109/L).10 Even if PCL can be considered as a particular form of EMM because of the involvement of the blood, PCL is a specific well-defined form of MM that should be excluded from the EMM spectrum. International guidelines and treatment recommendations have recently been published.10,11 (4) Lastly, SP is a PC dyscrasia characterized by a localized clonal PC infiltration in the absence of systemic tumor dissemination.12 In this case, the percentage of PCs in the bone marrow PC is <10%, the skeletal survey is normal, and CRAB symptoms are absent. Bone or extramedullary SPs are classified depending on the anatomical site involved. In SP, the presence of clonal bone marrow PCs (<10%) defines the subentity of SP with low marrow involvement and confers an adverse prognosis.5 Radiotherapy (>40 grays) is the treatment of choice and results in a high rate of local disease control (>80%) and a prolonged disease-free survival (nearly 30% for SP of the bone, and 50% to 65% for extramedullary plasmacytoma).12 Because SP does not meet the diagnostic criteria of MM, this entity should be excluded from the EMM spectrum.
Clinical entities of EMM reported in the myeloma literature
| EMM entities . | Definition . | Clinical presentation . |
|---|---|---|
| Bone-related plasmacytomas | Plasmacytomas developed from the bone, arising in continuity with the bone marrow. | Tumor masses affecting the axial skeleton: ribs, vertebrae, skull, sternum, pelvis. |
| Extramedullary disease | Soft-tissue plasmacytoma or PC infiltration of an anatomical site distant from the bone marrow. Secondary to a hematogenous spread. | Mainly affect the liver, skin, CNS, pleural effusion, kidneys, lymph nodes, pancreas. May be triggered by invasive procedures (ie, catheter insertion, surgical scars). |
| PCL | Aggressive variant of myeloma characterized by the presence of circulating plasma cells (>20% and/or absolute count >2 × 109/L). | Could be considered as EMM because of blood involvement. Extramedullary disease is also very common in PCL patients. |
| SP | Localized bone or extramedullary infiltration by clonal plasma cells without systemic tumor dissemination. | Bone marrow and skeletal survey are both normal. CRAB symptoms are absent. Focal radiotherapy is the treatment of choice. |
| EMM entities . | Definition . | Clinical presentation . |
|---|---|---|
| Bone-related plasmacytomas | Plasmacytomas developed from the bone, arising in continuity with the bone marrow. | Tumor masses affecting the axial skeleton: ribs, vertebrae, skull, sternum, pelvis. |
| Extramedullary disease | Soft-tissue plasmacytoma or PC infiltration of an anatomical site distant from the bone marrow. Secondary to a hematogenous spread. | Mainly affect the liver, skin, CNS, pleural effusion, kidneys, lymph nodes, pancreas. May be triggered by invasive procedures (ie, catheter insertion, surgical scars). |
| PCL | Aggressive variant of myeloma characterized by the presence of circulating plasma cells (>20% and/or absolute count >2 × 109/L). | Could be considered as EMM because of blood involvement. Extramedullary disease is also very common in PCL patients. |
| SP | Localized bone or extramedullary infiltration by clonal plasma cells without systemic tumor dissemination. | Bone marrow and skeletal survey are both normal. CRAB symptoms are absent. Focal radiotherapy is the treatment of choice. |
CNS, central nervous system; CRAB, hypercalcemia, renal failure, anemia, bone lesions; SP, solitary plasmacytoma.
In order to clarify the definition of EMM, Weinstock and Ghobrial have proposed that EMM should refer to purely extramedullary disease and so exclude bone-related plasmacytomas arising from the neighboring bone marrow.7 This proposal is based on the significant differences between the biological and prognostic characteristics of these 2 entities, as described in the following section. Thus, we also recommend restricting the definition of EMM to the presence of extramedullary disease in a patient with MM, excluding bone-related plasmacytomas, PCL, and SPs.
Biological and clinical features of EMM
The specific characteristics of EMM are summarized in Table 2. At the time of myeloma diagnosis, EMM is found in 6% to 8% of patients using a baseline staging that includes whole-body MRI and/or PET-CT.13-16 During the disease course, the prevalence of EMM increases, and EMM is found in 10% to 30% of patients.6,17 Importantly, extramedullary disease determined by PET-CT and/or MRI is not biopsy proven in most studies. Recent retrospective studies showed no increase in the incidence of EMM in patients treated with novel agents, including lenalidomide and bortezomib.17,18 However, a higher incidence of extramedullary relapse was reported in patients who underwent allogenic stem cell transplantation.19 In this respect, a recent retrospective study showed that the number of prior therapies and age were associated with a higher risk of extramedullary relapse, whereas the use of lenalidomide prior to the allogeneic transplant was found to be a protective factor.20
Summary of characteristics of EMM
| Characteristics . | Summary of features . |
|---|---|
| Definition | Soft-tissue plasmacytoma or PC infiltration of an anatomical site distant from the bone marrow (eg, strict extramedullary disease as defined in Table 1) |
| Incidence | 6% to 8% in de novo patients 10% to 30% in relapsed/refractory patients |
| Molecular pathogenesis | CD44high, CD56low, CXCR4/CXCL12 Hypoxia Ras, P53, FAK mutations |
| Clinical characteristics | Symptoms related to organ involvement Mostly liver, skin, CNS, pleural effusion, kidneys, lymph nodes, pancreas |
| Biological characteristics | High LDH, anemia, thrombocytopenia High-risk gene expression profile High-risk cytogenetics (17p deletion) |
| Morphology | Frequent immature/plasmablastic morphology |
| Staging | Value of PET-CT to detect extramedullary disease CNS EMM: MRI, CSF analysis (morphology, flow cytometry, protein electrophoresis) |
| Prognosis | EMM is an independent adverse prognostic factor in de novo MM patients receiving intensive therapy. Few series specifically analyzed the particular outcome of EMM. |
| Characteristics . | Summary of features . |
|---|---|
| Definition | Soft-tissue plasmacytoma or PC infiltration of an anatomical site distant from the bone marrow (eg, strict extramedullary disease as defined in Table 1) |
| Incidence | 6% to 8% in de novo patients 10% to 30% in relapsed/refractory patients |
| Molecular pathogenesis | CD44high, CD56low, CXCR4/CXCL12 Hypoxia Ras, P53, FAK mutations |
| Clinical characteristics | Symptoms related to organ involvement Mostly liver, skin, CNS, pleural effusion, kidneys, lymph nodes, pancreas |
| Biological characteristics | High LDH, anemia, thrombocytopenia High-risk gene expression profile High-risk cytogenetics (17p deletion) |
| Morphology | Frequent immature/plasmablastic morphology |
| Staging | Value of PET-CT to detect extramedullary disease CNS EMM: MRI, CSF analysis (morphology, flow cytometry, protein electrophoresis) |
| Prognosis | EMM is an independent adverse prognostic factor in de novo MM patients receiving intensive therapy. Few series specifically analyzed the particular outcome of EMM. |
CSF, cerebrospinal fluid; CXCL, CXC chemokine ligand; CXCR, CXC chemokine receptor; FAK, focal adhesion kinase; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PET-CT, positron emission tomography/computed tomography.
Unlike PCs from bone-related plasmacytomas, which have a mature/plasmacytic appearance, PCs from extramedullary plasmacytomas usually show an immature/plasmablastic morphology.6 High LDH levels, anemia, thrombocytopenia, nonsecretory MM, and high-risk cytogenetic features are more frequent in patients with de novo EMM than in patients with classic MM.16,21,22 Interestingly, the combination of high LDH level and high-risk cytogenetic features, parameters found to be more frequent in EMM, has been described to be associated with an ultrahigh risk of early progression or disease-related death in MM patients.23 Moreover, the frequency of high-risk gene expression profiles (MAF and PR) is found to be higher in EMM patients.24 In addition, patients with relapsed EMM may present with light-chain escape (ie, a shift from intact immunoglobulin [Ig] to free light-chain only secretion).25 Extramedullary disease PCs show a high frequency of p53 and Ras mutations and upregulation of a FAK.26-28 The molecular pathogenesis underlying the extramedullary spread of PCs is only partially understood. EMM PCs are characterized by a decreased expression of the CD56 adhesion molecule and an increased expression of CD44, which is involved in cell proliferation and migration.6,15,29 The increased expression of CXCR4 and its ligand CXCL12 have also been implied to contribute to the dissemination of PCs, notably through the activation of an epithelial-mesenchymal transition pattern.30-32 Moreover, hypoxia has been demonstrated as an important factor influencing the dissemination of PCs.33
In terms of clinical outcome, the presence of EMM at the time of diagnosis is associated with an adverse prognosis.16,24 At the time of relapse, extramedullary disease has an even worse prognosis with an overall survival of <6 months.34 This adverse prognosis is less pronounced in patients with bone-related plasmacytomas.34 This discrepancy argues for the restriction of the term EMM to extramedullary disease. EMM clinical situations are extraordinarily heterogeneous and their management is particularly challenging. With the following case-and-comment approach, we have aimed to describe the heterogeneity and principles of management of EMM.
Case 1: a relapsed myeloma patient with CNS involvement
Case presentation
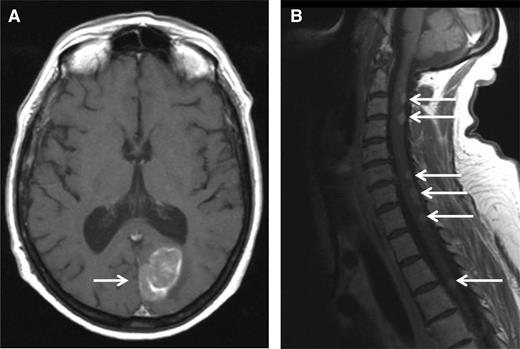
A 51-year-old woman was diagnosed with IgA-κ MM in 2004. At this time, she presented with symptomatic myeloma-related bone lesions, and a bone marrow aspirate confirmed the presence of 33% PCs. The International Scoring System (ISS) score was low (I), and a fluorescence in situ hybridization (FISH) analysis did not reveal any adverse cytogenetic factors (t[4;14] translocation, 17p deletion, and t[14;16] translocation). The first line of therapy consisted of 4 cycles of vincristine-adriamycin-dexamethasone followed by high-dose melphalan/ASCT. The patient achieved a complete remission (CR) after the completion of therapy, but the disease relapsed only 6 months after transplantation. At this time, the patient was treated with a regimen consisting of bortezomib-thalidomide-dexamethasone. During the fifth cycle of bortezomib-thalidomide-dexamethasone, she developed progressive ataxia, and an MRI scan revealed a paramedian occipital lesion (3.6 × 2 cm), as well as multiple posterior lesions of the medulla from C2 to T6 (Figure 1). A CSF analysis revealed the presence of 30 leukocytes per mm3, 80% of which were clonal PCs. At the same time, the percentage of bone marrow PCs was <5%, and no M-component was present in serum or urine. Therefore, we arrived at the diagnosis of a relapsed CNS EMM. The patient then started lenalidomide (25 mg/d, days 1-21) plus high-dose dexamethasone (40 mg/d, days 1-4; days 15-18) (Len-Dex) therapy in combination with cranial irradiation (30 grays) plus intrathecal (IT) injections of methotrexate (15 mg), cytarabine (40 mg), and hydrocortisone (20 mg) until the disappearance of PCs from the CSF (5 IT injections total). The clinical evolution was favorable with the disappearance of neurologic symptoms and the disappearance of both MRI lesions and CSF abnormalities. The patient continued to receive Len-Dex therapy, but the disease recurred after cycle 12 with the same CNS involvement. At that point, treatment was discontinued, and the patient died 3 weeks later.
MRI. MRI (T1 weighted) showing an occipital mass with leptomeningeal involvement (A, white arrow) and multiple posterior medullary lesions (B, white arrows), in a relapsed MM patient who developed progressive ataxia.
MRI. MRI (T1 weighted) showing an occipital mass with leptomeningeal involvement (A, white arrow) and multiple posterior medullary lesions (B, white arrows), in a relapsed MM patient who developed progressive ataxia.
Comment on patient 1
CNS involvement is uncommon and observed in ∼1% of MM patients.35,36 Retrospective studies highlight an extremely poor prognosis for CNS EMM with a median overall survival of <6 months.35-39 Any neurologic symptom presented by an MM patient should raise the possibility of the presence of CNS EMM. However, alternative diagnoses should be considered (eg, hypercalcemia, hyperviscosity, spinal cord compression, CNS infection, and peripheral neuropathy). MRI is the most sensitive imaging method to detect leptomeningeal infiltration. In the present case, MRI findings were strongly in favor of CNS EMM. CSF analysis is mandatory and should include the clonal characterization of PCs using both cytology and flow cytometry. FISH analysis of PCs from CSF is usually technically difficult. CSF analysis should also include protein measurement including IT M-component characterization by electrophoresis and immunofixation. Bacterial, viral, fungal, and/or parasitic screening should be considered for differential diagnosis. In the present case, the diagnosis was confirmed by the presence of clonal PCs in the CSF. Proving the diagnosis of CNS EMM can be challenging. For example, in case of an encephalic lesion detected by MRI along with an inconclusive CSF analysis, a surgical biopsy should be considered. However, the risk of the procedure has to be balanced with patient age, performance status, and the therapeutic impact. In addition, in case of a specific CNS-involvement symptom such as numb chin syndrome, we generally retain the diagnosis of CNS EMM, even if both MRI and the CSF analysis are not confirmative. Although the therapy of CNS EMM is difficult, the present case illustrates that a durable response can be achieved by using both systemic and CNS-specific therapy. Systemic CNS EMM therapy should ideally include drugs that may cross the blood-brain barrier (BBB). In addition to high-dose corticosteroids, thalidomide and lenalidomide have been reported to penetrate the BBB in nonhuman primates.40 In patients, thalidomide has been shown to cross the BBB.41 The third-generation IMiD pomalidomide has demonstrated a good penetrance of the BBB in a murine model.42 Notably, a durable CSF remission was recently reported using a pomalidomide-dexamethasone treatment.43 Of course, the ability of IMiDs to treat CNS myeloma should be evaluated in a larger number of patients, ideally in the context of prospective trials. With the exception of marizomib (in rodents),44 there is no clear evidence that PIs have the ability to cross the BBB. Alkylating agents (cyclophosphamide or melphalan) penetrate the CSF poorly. Other conventional chemotherapies known to cross the BBB (high-dose methotrexate or cytarabine) are not effective in treating MM. However, durable responses have been reported using bendamustine in combination with thalidomide.45 In addition to systemic anti-MM therapy, CNS irradiation and IT chemotherapy have been shown to improve the duration of response.35,37,38 Regarding CNS irradiation, both cranial and cranio-spinal irradiation are reported in the literature. However, larger and homogeneous series are needed to determine the best strategy. Overall, we recommend treating CNS EMM with the combination of (1) a systemic anti-MM regimen that crosses the BBB (ideally an IMiD-dexamethasone–based therapy), (2) CNS irradiation, and (3) IT chemotherapy until the disappearance of PCs from the CSF.
Case 2: patient with plasmacytoma of the pancreas and light-chain escape
Case presentation
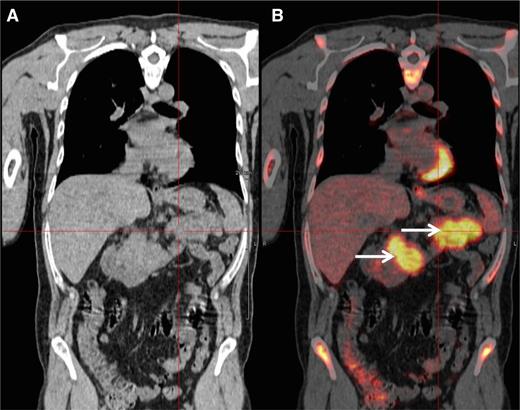
A 48-year-old man was diagnosed with IgG-κ myeloma in 2013. The patient presented with myeloma-related anemia and bone lesions. The prognostic analysis did not show the presence of high-risk FISH abnormalities, but a stage 3 ISS and an elevated LDH level. At this time no extramedullary disease was detected using PET-CT and MRI. The patient received induction therapy (3 courses of bortezomib-lenalidomide-dexamethasone), intensification (high-dose melphalan/ASCT), consolidation (2 courses bortezomib-lenalidomide-dexamethasone), and lenalidomide maintenance (IFM/DFCI2009 clinical trial). After completion of this sequence, the patient achieved a stringent CR (sCR). During cycle 10 of the maintenance therapy, the patient presented with epigastric abdominal pain and vomiting. Biochemistry tests showed elevated lipase levels, and a CT scan revealed a homogeneous hypertrophy of the pancreas. At the same time, the patient did not display any clinical criteria of myeloma relapse: he had normal blood cell counts and no CRAB symptoms. The bone marrow aspirate did not show abnormal plasmacytosis, and there was no M-spike in the serum electrophoresis. However, serum and urine κ free light-chain levels were elevated (250 mg/L and 300 mg/24 hours, respectively) confirming a light-chain escape. A PET-CT scan revealed an intense fluorodeoxyglucose (FDG) avidity of the pancreas (maximum standard uptake value = 11; Figure 2). The biopsy of the pancreas confirmed the infiltration by clonal PCs. Genetic and molecular analyses were performed and revealed no 17p deletion, no 14q32 recurrent translocation, and no BRAF mutation. The patient started pomalidomide-cyclophosphamide-dexamethasone therapy and achieved a CR according to standard criteria, including PET-CT. After 6 cycles, the patient is still continuing therapy.
PET-CT scan. PET-CT scan (A) demonstrating increased FDG avidity of the pancreas (B, arrows) in an MM patient who developed epigastric pain during maintenance therapy.
PET-CT scan. PET-CT scan (A) demonstrating increased FDG avidity of the pancreas (B, arrows) in an MM patient who developed epigastric pain during maintenance therapy.
Comment on patient 2
The present case illustrates the diagnostic and therapeutic approach to an EMM relapse. This high-risk MM patient (stage 3 ISS, elevated LDH) achieved an sCR after the completion of an intensive approach combining lenalidomide-bortezomib–based induction and consolidation, high-dose melphalan, and lenalidomide maintenance. During maintenance, he presented with abdominal symptoms that did not initially suggest an MM relapse. However, clinical tests revealed a pancreatic tumor, which should in this context be considered as a possible extramedullary manifestation. Moreover, in the present case, the hypothesis of extramedullary disease was supported by the light-chain escape, which could be a feature of EMM relapse.25 This particular type of relapse strongly supports the international guidelines that recommend the routine inclusion of Bence Jones proteinuria in the follow-up of MM patients.46 In this context of probable EMM, a PET-CT scan is a valuable tool to detect lesions with increased FDG avidity.47 In our daily practice, PET-CT imaging is commonly used in case of (1) clinical suspicion of EMM, (2) light-chain escape, and (3) staging and follow-up of nonsecretory MM. Whenever possible, a biopsy should be performed to confirm the diagnosis of extramedullary disease. Extramedullary relapse has a dismal prognosis with an overall survival of <6 months.34 As is usual, the therapeutic strategy should take into account the previous lines of treatment and the duration of response. In the present case, we started a pomalidomide-based triplet combination. Pomalidomide has demonstrated a response rate of ∼30% in EMM relapse.18 However, novel approaches are warranted to improve response rates and overall survival. The efficacy of monoclonal antibodies (eg, daratumumab and elotuzumab) in EMM has not been reported so far. Importantly, the molecular characterization of PCs may help to guide therapy. Indeed, BRAF mutation is a rare molecular event in MM (∼3% of patients) but seems to be more frequent in EMM patients.48 Andrulis et al reported a durable response using the BRAF inhibitor vemurafenib in an EMM patient harboring the BRAF V600E mutation.48 Because of this potential therapeutic impact, we check the presence of BRAF mutations in tissue samples of all patients with EMM relapse. Similarly, Heuck et al recently reported promising clinical responses using the mitogen-activated protein kinase inhibitor trametinib in relapsed MM patients harboring RAS or RAF mutations.49 Recently, immune therapies using autologous T cells expressing a tumor-specific chimeric antigen receptor have also shown promising responses (including a complete response) in relapsed EMM patients.50,51 Finally, radiotherapy of a soft-tissue plasmacytoma should always be considered to improve local disease control and analgesia.
Final considerations
EMM is associated with an adverse prognosis in newly diagnosed and in relapsing MM patients. Therefore, it is of clinical importance to optimize the detection of this entity. According to current international guidelines, only radiograph and MRI are recommended for the initial imaging staging.37,47,52 However, PET-CT is an effective tool to detect extramedullary disease, and the adverse prognostic impact of extramedullary disease assessed at diagnosis by PET-CT has been documented in several series.53 Indeed, some experts are already recommending PET-CT assessment for the initial staging and response assessment, especially in the context of intensive therapy.14,53
To the best of our knowledge, no prospective therapeutic studies have been specifically dedicated to EMM patients. Therefore, it is difficult to recommend a specific treatment strategy over another. In the future, subgroup analyses of large prospective trials focusing on EMM should be conducted to address this issue. Nevertheless, experts agree in considering de novo EMM as high-risk disease and recommend that patients are treated aggressively, if possible.54 Indeed, for de novo EMM patients eligible for stem cell transplantation, we propose a triplet induction therapy followed by high-dose melphalan/ASCT, a triplet consolidation therapy, and a maintenance treatment, consisting of at least lenalidomide. For high-risk patients, some colleagues propose the routine use of tandem ASCT.55 This proposal has been reinforced by the results of a pooled analysis of prospective studies suggesting the superiority of tandem ASCT in patients with poor prognostic features at diagnosis.56 Ongoing randomized trials are evaluating the addition of monoclonal antibodies during intensive up-front therapy, such as daratumumab (Cassiopea IFM-HOVON collaborative study) or elotuzumab (GMMG-HD6 study). These trials will use PET-CT at diagnosis and during patient follow-up and will therefore help in the definition of the optimal strategy for EMM patients.
For elderly MM patients not eligible for ASCT, bortezomib-melphalan-prednisone (VMP) or continuous Len-Dex are currently 2 of the most effective standards of care for up-front therapy. The impact of these 2 strategies on the outcome of EMM patients is currently unknown. A Spanish group recently reported the preliminary results of a phase 3 study evaluating the efficacy of an alternating VMP/Len-Dex sequence in de novo MM patients.57 This strategy demonstrated a high CR/sCR rate and an encouraging median progression-free survival of 28 months in high-risk MM patients. In this study, the outcome of standard-risk and high-risk patients did not differ significantly. However, these promising results should be interpreted with caution with regard to the number of patients and the median follow-up of the study. As in the case of young patients, ongoing phase 3 trials are also evaluating the addition of monoclonal antibodies to VMP (± daratumumab, Alcyone study) or continuous Len-Dex (± daratumumab, Maia study; ± elotuzumab, Eloquent-1 study).
At the time of relapse, there is no rationale to favor a specific therapeutic class (eg, IMiD, PI, or monoclonal antibody) over another. Previous lines of therapy and the duration of response should clearly be taken into account. Confirmation of the diagnosis of EMM through a biopsy is highly recommended and may provide the biological rationale for targeted therapy in some cases (eg, vemurafenib in BRAF mutated plasmacytoma). For CNS EMM, we strongly recommend the combination of CNS radiotherapy, IT chemotherapy, and systemic IMiD-based therapy. Innovative approaches using molecular targeted therapies48,49 or immune therapies (chimeric antigen receptor T cells)51 have recently shown promising results in a limited number of relapsed patients with EMM.
To conclude, EMM is a heterogeneous entity that affects ∼15% of MM patients during their overall disease course. Efforts should be made to optimally detect extramedullary disease. PET-CT could be an important tool at diagnosis and during follow-up. Future clinical trials should specifically include subgroup analyses to help define the optimal strategy for these high-risk patients. Nevertheless, the outcome of EMM patients remains exceedingly poor, and innovative strategies are warranted.
Acknowledgments
The authors gratefully acknowledge Dr Vincent Fleury (Nuclear Medicine Department, Nantes, France) for conducting the FDG-PET imaging.
Authorship
Contribution: C.T. and P.M. collected data, wrote and critically reviewed the manuscript, and gave final approval.
Conflict-of-interest disclosure: P.M. serves on the advisory boards of Celgene, Takeda, Amgen, Janssen, BMS, and Novartis. The remaining author declares no competing financial interests.
Correspondence: Philippe Moreau, Service d’hématologie Clinique, Centre Hospitalier Universitaire, Place Alexis Ricordeau, 44093 Nantes, France; e-mail: philippe.moreau@chu-nantes.fr.