As part of a year-long celebration in honor of Blood's 70th anniversary, we are publishing a series of editorials written by past Editors-in-Chief of the journal. The authors reflect on their experience at Blood in light of the journal's publication history. Each of these special pieces will highlight and discuss the impact of one or more original research articles that had a significant influence on the field or that mark a pioneering scientific development in hematology that appeared in the journal during the author's term as Editor-in-Chief.
I am delighted to have the opportunity to prepare this editorial in which I summarize the period from January 1, 1988, to December 31, 1992, during which I served as Editor-in-Chief of Blood. This interval was a time of substantial change for the journal. I was joined by an outstanding group of Associate Editors that included Bernard M. Babior, Edward J. Benz Jr, James D. Griffin, John H. Kersey, Tucker W. LeBien, Kenneth G. Mann, Theodore Zimmerman, and Neal S. Young. Following Dr Zimmerman’s untimely death in December of 1988, we were joined on the Associate Editor staff by Thomas F. Deuel.
The manuscripts published in the journal grew steadily during this period. There was significant diversity in the contents of the various manuscripts. For example, we published >80 manuscripts regarding the molecular genetics of malignant disorders. These studies included attempts to find a correlation between the specific breakpoint within the BCR-ABL cluster region rearrangement and the clinical severity of disease. One study not only concluded that there was no specific correlation of the laboratory and clinical features of chronic myeloid leukemia (CML) with specific breakpoints but also observed that chronic-phase duration was longer in patients with a particular breakpoint localization.1 In another study, the Philadelphia breakpoints were examined in acute lymphoblastic leukemia (ALL). These studies concluded that a rearrangement that had occurred in a multipotential stem cell actually gave rise to a variant of CML.2 Another subset of cases had a de novo ALL mutation which had occurred in a committed lymphoid progenitor.3 Other mapping studies defined breakpoints in other myeloid disorders.4 Many studies also identified single nucleotide changes in proto-oncogenes which could be implicated in the carcinogenic mechanism.5-7
During the 5-year interval, we published >80 manuscripts pertaining to hematopoietic growth factors, including those that are capable of inducing fetal hemoglobin in erythroid progenitors.8 Many studies were focused on attempts to unravel the mechanism of erythropoietin regulation9 and the regulatory elements of the erythropoietin gene.10 Many other studies of recombinant hematopoietic growth factors were described; in addition, the observation that megakaryocytopoiesis could be stimulated in experimental animals by interleukin-6 was made.11
There were >60 articles published related to the molecular genetics of the thalassemias. For example, deletions upstream from the α gene coding sequences which remained intact were found to have eliminated hypersensitive sites leading to silencing of the linked α-globin genes (Figure 1).12,13 Thus, these hypersensitive sites are clearly involved in regulation of the α-globin genes. Many studies also focused on the molecular mechanisms of the β-thalassemias. These included correlation of specific mutations with the clinical phenotype, whether thalassemia major or thalassemia intermedia.14 Novel mutations in the promoter regions of the γ-globin genes in individuals with hereditary persistence of fetal hemoglobin (HPFH) identified regulatory elements and gave insight into their molecular mechanisms.15 HPFH can also arise due to deletions that remove the β-globin gene.16 Other studies focused on treatment of severe β-thalassemia, including transfusion and administration of erythropoietin and/or iron chelators.17
Deletions 5′ to the α-globin gene cluster associated with α-thalassemia. A map of the human α-globin gene cluster and 5′ flanking region is shown on the first line with genes and coordinates as defined above. The positions of the DNase l hypersensitive sites (HS) are shown by the vertical arrows with the size of the arrow proportionate to the degree of sensitivity. The telomere of chromosome 16q is shown at the extreme left of the diagram (Tel). Below the map are the 4 described deletions covering the HS region and associated with α-thalassemia. The dark bars represent the regions deleted in each case with (- - - - -) denoting undefined extent. The 4 deletions are RA, TI, IJ, and MM (present report). Reprinted from Romao et al12 with permission.
Deletions 5′ to the α-globin gene cluster associated with α-thalassemia. A map of the human α-globin gene cluster and 5′ flanking region is shown on the first line with genes and coordinates as defined above. The positions of the DNase l hypersensitive sites (HS) are shown by the vertical arrows with the size of the arrow proportionate to the degree of sensitivity. The telomere of chromosome 16q is shown at the extreme left of the diagram (Tel). Below the map are the 4 described deletions covering the HS region and associated with α-thalassemia. The dark bars represent the regions deleted in each case with (- - - - -) denoting undefined extent. The 4 deletions are RA, TI, IJ, and MM (present report). Reprinted from Romao et al12 with permission.
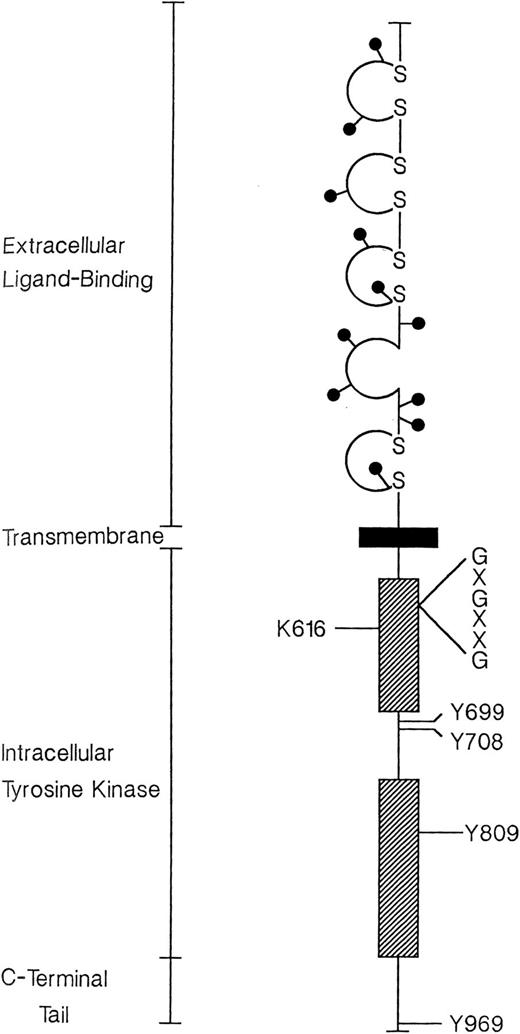
We also focused on publishing review articles in nearly every issue of the journal, with occasional editorial commentaries on specific manuscripts. For example, in the January 1, 1999, edition, we published the review “Colony-stimulating factor-1 receptor” by Charles Sherr in which the functional domains of macrophage–colony-stimulating factor receptor were defined (Figure 2).18 The reviews were designed to educate our readership and often focused on clinical topics of relevance to practicing clinicians. For example, the May 15, 1992, issue included an editorial on “Hydroxyurea: specific therapy for sickle cell anemia?”19 This issue also included a basic science review of the molecular genetics of von Willebrand disease.20
Predicted structure of human CSF-1R. The extracellular ligand-binding portion is organized into 5 immunoglobulin-like domains, 4 of which are stabilized by disulfide bonds (S-S). All such domains are homologous to sequences of the C2-SET. Positions of asparagine-linked oligosaccharide chains are indicated (0). The intracellular kinase domain (stippled bars) is interrupted by spacer sequences containing 2 predicted sites of receptor phosphorylation at tyrosines (Y) 699 and 708. A third phosphorylation site (Y809) occurs in the distal core consensus sequences. The membrane-proximal kinase segment includes a glycine-rich signature sequence (G-X-G-X-X-G) characteristic of kinases in general. This is followed by the ATP-binding site at lysine (K) 616. The C-terminal tail contains a single tyrosine residue (Y969) whose removal upregulates receptor kinase activity. The data are taken from a previously reported human c-fms complementary DNA sequence. Unpublished information regarding sites of phosphorylation within the murine receptor was provided by Drs P. Tapley, A. Kazlauskas, and L. R. Rohrschneider and was aligned with the human amino acid sequence. Reprinted from Sherr18 with permission.
Predicted structure of human CSF-1R. The extracellular ligand-binding portion is organized into 5 immunoglobulin-like domains, 4 of which are stabilized by disulfide bonds (S-S). All such domains are homologous to sequences of the C2-SET. Positions of asparagine-linked oligosaccharide chains are indicated (0). The intracellular kinase domain (stippled bars) is interrupted by spacer sequences containing 2 predicted sites of receptor phosphorylation at tyrosines (Y) 699 and 708. A third phosphorylation site (Y809) occurs in the distal core consensus sequences. The membrane-proximal kinase segment includes a glycine-rich signature sequence (G-X-G-X-X-G) characteristic of kinases in general. This is followed by the ATP-binding site at lysine (K) 616. The C-terminal tail contains a single tyrosine residue (Y969) whose removal upregulates receptor kinase activity. The data are taken from a previously reported human c-fms complementary DNA sequence. Unpublished information regarding sites of phosphorylation within the murine receptor was provided by Drs P. Tapley, A. Kazlauskas, and L. R. Rohrschneider and was aligned with the human amino acid sequence. Reprinted from Sherr18 with permission.
A number of specific changes were also made in the organization of the journal. Beginning in January 1989, the table of contents was divided into categories of specific papers to facilitate rapid review of the content of each issue. In 1990, we began semimonthly publication, a reflection of the continued growth of the journal and the desire to communicate manuscripts as rapidly as possible.
This interval was also a period of significant change for the journal. When I began my editorship, Blood was published by Grune & Stratton, which held title to the journal. The income to the American Society of Hematology (ASH) was relatively small. Subsequently, Grune & Stratton was merged into Saunders, which retained title. However, during this 5-year period, H. Franklin Bunn, who served as the Chair of the ASH Publications Committee, worked tirelessly on behalf of ASH to improve the financial relationship between ASH and the publisher. Ultimately, a contract was negotiated which permitted ASH to gain title to the journal over a 5-year period. As a consequence of this negotiation and the newly defined relationship, the income from the journal to ASH rose by >10-fold. Certainly, at that time, the journal was the major source of income for ASH and permitted many of its activities to be conducted to the benefit of its membership. Ultimately, ASH assumed responsibility for publication of the journal, a status which continues currently.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal