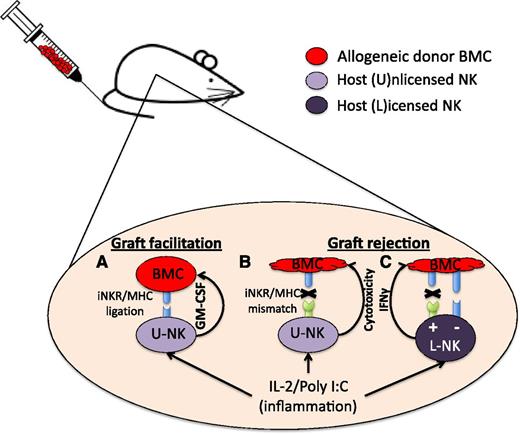
In this issue of Blood, Alvarez et al describe a novel inhibitory receptor-mediated role for unlicensed natural killer (U-NK) cells in allogeneic graft facilitation.1
In the mouse allogeneic transplant setting, inflammatory cytokines prime NK cells to enhance their function so that U-NK cells containing inhibitory receptors matching cognate MHCI ligand in the graft secrete GM-CSF, which facilitates donor cell engraftment (A). U-NK cells whose inhibitory receptors do not recognize MHCI ligands on the graft (thus mismatched) drive graft rejection when previously primed (B). L-NK cells not encountering cognate MHCI ligands (missing self) mediate graft rejection (+), whereas tolerance (−) results if L-NK cells encounter cognate MHCI ligands (C). BMC, bone marrow cell; IFNγ, interferon-γ; IL-2, interleukin-2; iNKR, inhibitory NK cell receptor.
In the mouse allogeneic transplant setting, inflammatory cytokines prime NK cells to enhance their function so that U-NK cells containing inhibitory receptors matching cognate MHCI ligand in the graft secrete GM-CSF, which facilitates donor cell engraftment (A). U-NK cells whose inhibitory receptors do not recognize MHCI ligands on the graft (thus mismatched) drive graft rejection when previously primed (B). L-NK cells not encountering cognate MHCI ligands (missing self) mediate graft rejection (+), whereas tolerance (−) results if L-NK cells encounter cognate MHCI ligands (C). BMC, bone marrow cell; IFNγ, interferon-γ; IL-2, interleukin-2; iNKR, inhibitory NK cell receptor.
It is well know that NK cells are part of the innate immune system and play a scavenger role to detect targets marked by “missing self” induced by viral infection or malignant transformation. Classically, NK cells are thought to function mainly by elimination of targets through direct cell-cell–mediated cytotoxicity and production of inflammatory cytokines to recruit and activate other immune effectors. However, some NK cell subsets are hyporesponsive and have diminished function against targets. NK cell function is mediated by a process termed licensing, also referred to as education, arming, or tuning, which is defined as the event through which NK cells acquire functional maturation through signaling via an inhibitory receptor on the NK cell after ligation with the cognate major histocompatibility complex I (MHCI) ligand.2 These paradigms are the same in mice and humans. NK cells that do not receive a licensing signal, termed U-NK cells, were originally thought to be hyporesponsive in terms of direct cytotoxicity and immune-recruiting interferon-γ production, raising questions about the purpose of these cells. Recent studies have elucidated possible roles for this population as we better understand mechanisms of NK-cell activation. For instance, hyporesponsiveness in U-NK cells can be overcome by cytokine activation. In the transplant setting, this activation can lead to graft rejection similar to that elicited by licensed NK (L-NK) cells.3 Cytokines can also prime U-NK cells to induce expression of inhibitory receptors, promoting maturation and de novo licensing.4 In the setting of mouse cytomegalovirus infection, unlicensed NK cells are a key component of the immune response.5 Additionally, therapeutic antibodies can override hyporesponsiveness in U-NK cells by robust signaling through CD16 (FcRγIII), which yields potent activation in the autologous setting in the absence of inhibitory killer-immunoglobulin receptor engagement with cognate HLA ligand.6 Together these studies show that in settings of physiological stress, such as viral infections or tumor formation, U-NK cells can be induced to function similar to or even superior to L-NK cells.
Alvarez and colleagues report a new role for U-NK cells, specifically allogeneic graft facilitation. After allogeneic transplantation, host U-NK cells, that receive inhibitory receptor signals triggered by cognate ligand expressed on cells in the graft, produce granulocyte macrophage colony-stimulating factor (GM-CSF) facilitating engraftment (see figure). This process is Scr homology protein tyrosine phosphatase-I (SHP-1) dependent, enhanced with prior activation/inflammation, and is not seen in syngeneic (autologous) settings. Although clearly a novel role for U-NK cells, it raises the question as to the physiological context where U-NK cells encounter cognate MHC ligand and why they secrete GM-CSF under such conditions. The only physiological condition where a women lives with a foreign haploidentical (HLA half-matched) fetal graft capable of providing cognate MHC ligands to U-NK cells is pregnancy. NK cells represent the primary lymphocyte infiltrating the pregnant decidua7 and play a critical role in supporting placental growth.8 Decidual NK cells do this through secretion of growth factors rather than cytotoxic functions. Human decidual NK cells express high levels of inhibitory receptors, and impaired maternal-receptor/fetal-HLA interactions can result in increased risk during pregnancy.9 This allows one to envision situations under which unlicensed decidual NK cells could encounter licensing ligands. Conversely, U-NK cell–mediated secretion of GM-CSF would be beneficial in pregnancy as this cytokine has a pivotal role in embryo implantation and development.10 Taken together, one could speculate that the findings in the Alvarez study describe an evolutionary role for U-NK cells in embryo implantation.
These findings are of translational importance for clinical transplantation. It is well described clinically that potent lymphocyte depletion (NK and T cells) methods lead to a higher incidence of graft failure. The Alvarez study highlights the benefits of U-NK cells in the allogeneic transplant setting but does raise some questions. First, in human transplantation using a myeloablative preparative regimen, few host NK cells survive, thus direct extension of these findings is perhaps most relevant to reduced-intensity conditioning. In addition, unlicensed NK cells in the graft may actually play a similar role by encountering cognate ligand in the host as class I molecules are also displayed on nonhematopoietic tissue. The Alvarez data suggest that these findings may be most relevant in HLA-mismatched transplant. Because NK cell inhibitory receptors evolve independently of MHC receptors (they are encoded on different chromosomes), it is expected that all mice and humans have variable repertoires of U-NK cells capable of mediating a dominant GM-CSF response to MHC differences. Transplant strategies that exploit the novel role of U-NK cells in the HLA-mismatched allogeneic setting, or strategies to mimic NK cell inhibitory receptor ligation by cognate ligand, in combination with preactivation with cytokine administration may lead to novel treatments to facilitate engraftment after hematopoietic transplantation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.