Key Points
Maternal platelet count response was not different for IVIg and corticosteroids in this retrospective study of pregnant women with ITP.
Neonatal outcomes were overall favorable and similar after treatment of maternal ITP with IVIg or corticosteroids.
Abstract
Treatment options for immune thrombocytopenia (ITP) in pregnancy are limited, and evidence to guide management decisions is lacking. This retrospective study of singleton pregnancies from 2 tertiary centers compared the effectiveness of intravenous immunoglobulin (IVIg) and corticosteroids in treatment of ITP. Data from 195 women who had 235 pregnancies were reviewed. Treatment was not required in 137 pregnancies (58%). Of the remaining 98 pregnancies in 91 women, 47 (48%) were treated with IVIg and 51 were treated with corticosteroids as the initial intervention. Mean maternal platelet count at birth did not differ between groups (IVIg 69 × 109/L vs corticosteroids 77 × 109/L; P = .71) nor did the proportion of mothers who achieved a platelet count response (IVIg 38% vs corticosteroids 39%; P = .85). There were no fatal or severe maternal, fetal, or neonatal hemorrhages. Of 203 neonates in whom platelet counts were available, 56 (28%) had a birth platelet count <150 × 109/L and 18 (9%) had platelet counts <50 × 109/L. Nadir platelet counts for most affected neonates occurred at birth, although for some neonates, nadir platelet counts occurred up to 6 days postnatally. Intracranial hemorrhage was noted in 2 neonates (nadir platelet counts were 135 and 18 × 109/L). There were no neonatal deaths. The majority of pregnant women with a history of ITP did not require treatment, and neonatal outcomes were comparable for mothers who received IVIg or corticosteroids for treatment of maternal ITP.
Introduction
Immune thrombocytopenia purpura (ITP) affects 1-10 in 10 000 pregnancies.1 Most women with ITP have mild to moderate thrombocytopenia, and ∼ 30% to 35% require intervention during pregnancy.2,3 Similar to the treatment of ITP in the nonpregnant individual, both corticosteroids and intravenous immunoglobulin (IVIg) are acceptable treatments for ITP in pregnancy.4,5 The evidence for effectiveness of corticosteroids and IVIg, however, is largely extrapolated from nonpregnant patients with ITP and has not been adequately assessed in pregnancy.4 Whereas IVIg has been associated with adverse reactions, including headaches, meningeal irritation, and hemolytic anemia in the pregnant and nonpregnant individual,6 corticosteroid use during pregnancy has been linked with the development of gestational diabetes,7 and fetal orofacial clefts with exposure during the first trimester.8,9 Thus, the objective of this study was to compare the efficacy of IVIg and corticosteroids on maternal ITP.
Methods
A retrospective study of pregnant women carrying singletons, with ITP predating the pregnancy or diagnosed therein, was conducted in 2 tertiary care centers in Canada: Mount Sinai Hospital in Toronto and McMaster University Medical Centre in Hamilton, from January 2000 to August 2014. The study was approved by the research ethics boards at each hospital and conducted with informed consent. Patients were identified through hospital medical records, using International Statistical Classification of Diseases, 10 revision codes for thrombocytopenia, and cross-referenced with electronic databases. Subsequent pregnancies from the same women were included, as long as eligibility criteria were met for each individual pregnancy. Electronic medical records and paper charts were reviewed independently at each site by 2 investigators (D.S. and S.G.). Thrombocytopenia of an alternate etiology, including preeclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), and sepsis, led to exclusion. The diagnosis of ITP was confirmed if patients had a prior history of thrombocytopenia, without another etiology. Patients whose platelet counts remained above 70 × 109/L in pregnancy and normalized in the postpartum period were deemed to have gestational thrombocytopenia and were excluded.10
The primary outcomes for this study were the maternal platelet count at delivery and response to treatment. Complete response was defined as a maternal platelet count of 100 × 109/L or more and partial response as a maternal platelet count between 30 and 100 × 109/L and an increase in platelet count of twice the baseline value.11
We considered the following secondary outcomes:
A maternal composite outcome of postpartum hemorrhage, predelivery platelet transfusion, peripartum transfusion of any blood product, or postpartum reduction in the hemoglobin concentration of 30 g/L or more
Maternal bleeding, classified according to the International Society of Thrombosis and Hemostasis as: (a) fatal bleeding, (b) symptomatic bleeding in a critical area or organ (intracranial, intraspinal, or retroperitoneal), (c) a reduction in hemoglobin concentration of 30 g/L or more or leading to transfusion of packed red blood cells, and/or (d) vaginal bleeding leading to fetal distress, hospitalization, or birth before 38 weeks’ gestation12,13 ; or postpartum hemorrhage defined as an estimated blood loss of 500 mL or more following vaginal delivery or 1000 mL or more following cesarean section14
A fetal/neonatal composite outcome of the following adverse events: stillbirth, preterm birth before 34 weeks of gestation, small for gestational age size (birth weight <10th percentile for gestational age), or Apgar score <7 at 5 minutes
Neonatal platelet counts at birth
Neonatal intracranial hemorrhage diagnosed before hospital discharge
Mode of delivery
Statistical analysis
Baseline characteristics of the study population were summarized using descriptive statistical methods. To examine the impact of the treatments, patient characteristics and outcomes were compared among 3 groups (ie, no treatment, IVIg, corticosteroids) using χ2 test or Fisher exact test as appropriate for categorical variables, and analysis of variance (F test) or Kruskal-Wallis test as appropriate for continuous variables. Either the Student t test or Wilcoxon rank sum test was used for the comparisons of continuous variables between 2 treatment groups, as appropriate. Highly skewed data were first normalized using log-transformation if applicable. To account for the possible impact of correlated or clustered data owing to repeat pregnancies from the same mother, logistic or linear regression models with the generalized estimating equation (GEE) approach were applied. Similar methods were also used in the post-hoc analysis comparing outcomes between IVIg and corticosteroids in the subgroup of patients with a history of maternal splenectomy. Data management and statistical analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC) and R 2.15 (www.r-project.org). A 2-sided significance level of 0.05 was used, without adjustment for multiple comparisons.
Results
A total of 689 pregnancies with maternal thrombocytopenia (platelets <100 × 109/L), or history of ITP, were identified during the study interval at both centers. Of these, 416 pregnancies were excluded because of thrombocytopenia secondary to another etiology, 4 due to multiple gestation, 4 owing to simultaneous treatment with IVIg and corticosteroids, and 30 because of crossover between treatment groups in repeat pregnancies. The latter 2 exclusions were necessary to allow for statistical analysis using the GEE approach to account for possible effects of clustered data owing to repeat pregnancies from the same mother. Thus, 235 pregnancies in 195 women were included, of whom 161 (83%), 28 (14%), and 6 (3%) had 1, 2, or 3 pregnancies, respectively, during the study period. Intervention was not introduced in 137 pregnancies (58%). Of the remaining 98 pregnancies (in 91 women), IVIg was the initial treatment choice in 47 pregnancies (48%) and corticosteroids in 51, with selection of the initial agent dependent on physician choice.
The mean (standard deviation [SD]) dose of IVIg, when used as initial treatment, was 1.0 g/kg (0.22). Corticosteroids as initial treatment included prednisone in 46 pregnancies (92%), dexamethasone in 4 pregnancies (7.8%), and both prednisone and dexamethasone in 1 pregnancy (2%). The median initial prednisone dose was 50 mg/d (interquartile range [IQR] 35-75 mg/d), translating to a mean (SD) of 0.65 mg/kg (0.3), with a range of 0.1-1.2 mg/kg/d. Of 44 patients treated with prednisone as the initial agent for whom milligram per kilogram dosing was available (missing in 2), 33 (75%) received prednisone 0.5-1.0 mg/kg/d for a median duration of 10 days (IQR 7-18 days). Dexamethasone was used at 40 mg/d in all 4 pregnancies; for 4 and 5 days in each of 2 pregnancies and not documented in 2 others.
Of pregnancies treated with IVIg at any point in gestation, adverse events were reported in 7/53 (13%) and included hemolytic anemia in 1 (2%), headache in 3 (6%), and other in 3 (6%), encompassing 1 instance each of swelling, flushing, and chills/rigors plus light-headedness. One of the instances where headache was reported was accompanied by nausea and fever.
Of pregnancies treated with corticosteroids at any point in gestation, adverse events were reported in 9/67 (13%), including hyperglycemia requiring treatment in 6 (9%), hyperglycemia with neonatal hypoglycemia in 1 (2%), infection in 1 (2%), and other in 1 (2%), encompassing insomnia and jitteriness.
Maternal characteristics
The characteristics of included pregnancies are shown in Table 1. Significant differences were not observed among the 3 groups with regard to maternal body mass index, age at ITP diagnosis, gestational age at platelet count nadir, or mode of delivery. Overall, differences in platelet count–related variables were identified in the “nontreatment” group compared with the “treatment group”; however, no significant differences were observed between the 2 treatment groups.
Treatment-specific cohort characteristics
| . | No treatment (n = 137) . | IVIg (n = 47) . | Corticosteroids (n = 51) . | P value . | |
|---|---|---|---|---|---|
| All groups . | IVIg vs corticosteroids . | ||||
| Maternal age (y), mean (SD) | 32.7 (4.1) | 31.1 (4.8) | 30.5 (5.1) | .01 | .52 |
| Nulliparity (n), (%) | 47 (34.3) | 27 (57.5) | 26 (51.0) | .01 | .52 |
| BMI (kg/m2), mean (SD) | 27.9 (6.3) | 27.9 (6.7) | 28.5 (5.8) | .92 | .70 |
| Age at ITP diagnosis, mean (SD) | 24.9 (7.2) | 27.2 (6.5) | 26.7 (6.0) | .10 | .72 |
| Maternal pretreatment platelet count (×109/L), mean (SD) | NA | 49 (25) | 50 (22) | — | .80 |
| Neonatal platelet nadir (×109/L), mean (SD) | 106 (76) | 35 (21) | 43 (26) | <.0001 | .11 |
| Gestational age at platelet nadir (wk), median (IQR) | 36.0 (27.3-38.0) | 36.0 (30.0-38.0) | 34 (28.8-37.0) | .31 | .18 |
| Operative vaginal* delivery (n/N), (%) | 9/106 (8.5) | 1/35 (2.9) | 1/43 (2.3) | .38 | .99 |
| Cesarean section, n (%) | 53 (38.7) | 20 (42.6) | 15 (29.4) | .36 | .17 |
| . | No treatment (n = 137) . | IVIg (n = 47) . | Corticosteroids (n = 51) . | P value . | |
|---|---|---|---|---|---|
| All groups . | IVIg vs corticosteroids . | ||||
| Maternal age (y), mean (SD) | 32.7 (4.1) | 31.1 (4.8) | 30.5 (5.1) | .01 | .52 |
| Nulliparity (n), (%) | 47 (34.3) | 27 (57.5) | 26 (51.0) | .01 | .52 |
| BMI (kg/m2), mean (SD) | 27.9 (6.3) | 27.9 (6.7) | 28.5 (5.8) | .92 | .70 |
| Age at ITP diagnosis, mean (SD) | 24.9 (7.2) | 27.2 (6.5) | 26.7 (6.0) | .10 | .72 |
| Maternal pretreatment platelet count (×109/L), mean (SD) | NA | 49 (25) | 50 (22) | — | .80 |
| Neonatal platelet nadir (×109/L), mean (SD) | 106 (76) | 35 (21) | 43 (26) | <.0001 | .11 |
| Gestational age at platelet nadir (wk), median (IQR) | 36.0 (27.3-38.0) | 36.0 (30.0-38.0) | 34 (28.8-37.0) | .31 | .18 |
| Operative vaginal* delivery (n/N), (%) | 9/106 (8.5) | 1/35 (2.9) | 1/43 (2.3) | .38 | .99 |
| Cesarean section, n (%) | 53 (38.7) | 20 (42.6) | 15 (29.4) | .36 | .17 |
BMI, body mass index; NA, not applicable.
Operative vaginal deliveries were carried out by vacuum extraction in all but 1 case in the nontreatment group, in which forceps were used.
Antepartum hemorrhage was observed in 2 (0.7%) pregnancies from the nontreatment group, 5 (10.6%) from the IVIg group (where 3 pregnancies started treatment prior to the bleeding event and 2 started treatment on the day of the bleeding event), and 3 (5.9%) from the corticosteroid group (where 1 started treatment before and 2 started on the day of the bleeding event). In 2 pregnancies, bleeding resulted in preterm birth, and in both this was as a result of an obstetric event (placenta previa in 1 pregnancy and placental abruption in the other).
Neonatal characteristics
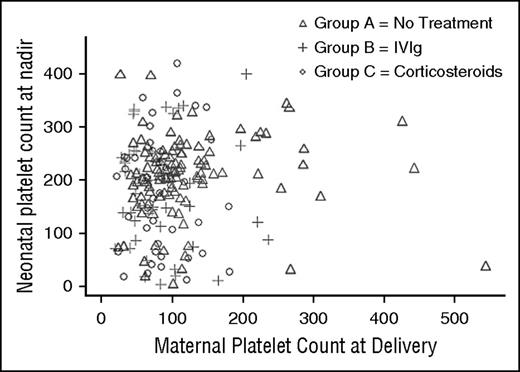
Two hundred thirty-one neonates were live births and 4 were intrauterine deaths. At least 1 postnatal platelet count was available for 203 (88%) live-born neonates. Of these, 56 (28%) had a platelet count <150 × 109/L and 18 (9%) had a platelet count <50 × 109/L. There was no correlation between maternal platelet count at delivery and the nadir neonatal platelet count (correlation coefficient 0.06 [0.39] for all groups and −0.02 [0.84] for IVIg compared with prednisone; Figure 1).
Neonatal platelet count nadir compared with maternal platelet count at delivery by treatment regimen demonstrating a lack of relationship between the variables.
Neonatal platelet count nadir compared with maternal platelet count at delivery by treatment regimen demonstrating a lack of relationship between the variables.
Cord blood platelet counts were measured in 167 neonates (72%). Although cord platelet counts were available for 85 neonates (89%) whose mothers received treatment, they were available for only 82 neonates (61%) whose mothers did not require treatment. Overall, cord platelet counts <150 × 109/L were observed in 38/231 (23%) neonates, and specifically in 18/109 neonates (17%) whose mothers were not treated. Of the 129 neonates with normal cord platelet counts, a repeat platelet count was available within 48 hours in 84 (65%). Platelet counts <150 × 109/L (range of 45-149) occurred in 9 (11%). The nadir neonatal platelet count occurred in the cord sample in 30% of neonates, although it was encountered as late as day 6 postnatally in 2 (4%).
Of the 14 neonates in whom platelet count nadir was reached at or beyond 3 days of life, 3 were premature. The infant with a platelet count nadir of 66 at day 6 of life had a cord platelet count of 144 × 109/L and was born in the late preterm period (35 weeks’ gestation). The infant with a platelet count nadir of 49 × 109/L on day 4 of life had an initial platelet count of 137 × 109/L, was born at 25 weeks’ gestation, and was growth restricted. The third neonate, with a platelet count nadir of 46 × 109/L on day 4 of life following an initial platelet count of 76 × 109/L, was born at 31 weeks’ gestation. This infant’s neonatal intensive care unit course was uncomplicated, and the discharge note attributed neonatal thrombocytopenia to the maternal condition. The remaining neonates with a platelet count nadir beyond 3 days of life were all born at term. None of the neonates with a platelet count nadir at or beyond 3 days of life had sepsis.
Operative vaginal delivery (assistance with forceps or vacuum) was needed for the birth of 11 neonates, none of whom had bleeding complications. Nine of the neonates were born to mothers who did not require treatment of ITP during that pregnancy. Cord platelet counts were not available for 6 of these neonates, whereas in 1, the cord platelet count was 121 × 109/L, with a nadir of 80 × 109/L on day 2 of life.
Primary and secondary outcomes
The primary and secondary outcomes are presented in Table 2. There were no maternal fatalities. No differences between the IVIg and corticosteroid groups were observed with regard to the primary and a majority of secondary outcomes. A significant difference in the maternal composite outcome was noted favoring the prednisone group (Table 2). A trend toward a higher use of peripartum transfusion of any blood product was observed in the IVIg group compared with the corticosteroid group (9 vs 3; P = .05). There was no significant difference in the fetal composite outcome between the IVIg and corticosteroid groups (Table 2).
Maternal and neonatal outcomes according to treatment strategy
| Outcomes . | No treatment (n = 137) . | IVIg (n = 47) . | Corticosteroids (n = 51) . | P value . | |
|---|---|---|---|---|---|
| All groups . | IVIg vs corticosteroids . | ||||
| Maternal response to initial treatment (n), (%) | N/A | 18 (38) | 20 (39) | — | .85 |
| Maternal platelet count at delivery (×109/L), mean (SD) | 103.2 (1.8) | 68.7 (1.8) | 77.3 (1.6) | <.0001 | .71 |
| Antepartum hemorrhage (n), (%) | 2 (1.5) | 5 (10.6) | 3 (5/9) | .08 | .39 |
| Postpartum hemorrhage (n), (%) | 6 (4.4) | 9 (19.2) | 6 (11.8) | <.03 | .33 |
| Pre-delivery platelet transfusion (n), (%) | 1 (0.7) | 6 (12.8) | 3 (5.9) | <.02 | .25 |
| Peripartum transfusion: any blood product (n), (%) | 2 (1.5) | 9 (19.2) | 3 (5.9) | .01 | .05 |
| Haemoglobin drop >30 g/L after delivery (n/N), (%) | 9/106 (8.5) | 12/47 (25.5) | 9/47 (19.2) | .02 | .39 |
| Stillbirth (n), (%) | 2 (1.5) | 0 | 2 (3.9) | .37 | .49 |
| Preterm birth <37 wk (n), (%) | 16 (11.7) | 4 (8.5) | 5 (9.8) | .81 | .99 |
| Preterm birth <34 wk (n), (%) | 7 (5.1) | 2 (4.3) | 1 (2.0) | .63 | .61 |
| Birth weight (g), mean (SD) | 3309 (637) | 3193 (769) | 3308 (521) | .54 | .38 |
| Small for gestational age (n/N), (%) | 6/136 (4.4) | 6/47 (12.8) | 5/51 (9.8) | .12 | .64 |
| Apgar score <7 at 5 min (n), (%) | 5 (3.7) | 2 (4.3) | 3 (5.9) | .80 | .99 |
| Maternal composite outcome (n), (%) | 14 (10.2) | 22 (46.8) | 12 (23.5) | <.0001 | .02 |
| Fetal/neonatal composite outcome (n), (%) | 13 (9.5) | 9 (19.2) | 9 (17.7) | .17 | .87 |
| Outcomes . | No treatment (n = 137) . | IVIg (n = 47) . | Corticosteroids (n = 51) . | P value . | |
|---|---|---|---|---|---|
| All groups . | IVIg vs corticosteroids . | ||||
| Maternal response to initial treatment (n), (%) | N/A | 18 (38) | 20 (39) | — | .85 |
| Maternal platelet count at delivery (×109/L), mean (SD) | 103.2 (1.8) | 68.7 (1.8) | 77.3 (1.6) | <.0001 | .71 |
| Antepartum hemorrhage (n), (%) | 2 (1.5) | 5 (10.6) | 3 (5/9) | .08 | .39 |
| Postpartum hemorrhage (n), (%) | 6 (4.4) | 9 (19.2) | 6 (11.8) | <.03 | .33 |
| Pre-delivery platelet transfusion (n), (%) | 1 (0.7) | 6 (12.8) | 3 (5.9) | <.02 | .25 |
| Peripartum transfusion: any blood product (n), (%) | 2 (1.5) | 9 (19.2) | 3 (5.9) | .01 | .05 |
| Haemoglobin drop >30 g/L after delivery (n/N), (%) | 9/106 (8.5) | 12/47 (25.5) | 9/47 (19.2) | .02 | .39 |
| Stillbirth (n), (%) | 2 (1.5) | 0 | 2 (3.9) | .37 | .49 |
| Preterm birth <37 wk (n), (%) | 16 (11.7) | 4 (8.5) | 5 (9.8) | .81 | .99 |
| Preterm birth <34 wk (n), (%) | 7 (5.1) | 2 (4.3) | 1 (2.0) | .63 | .61 |
| Birth weight (g), mean (SD) | 3309 (637) | 3193 (769) | 3308 (521) | .54 | .38 |
| Small for gestational age (n/N), (%) | 6/136 (4.4) | 6/47 (12.8) | 5/51 (9.8) | .12 | .64 |
| Apgar score <7 at 5 min (n), (%) | 5 (3.7) | 2 (4.3) | 3 (5.9) | .80 | .99 |
| Maternal composite outcome (n), (%) | 14 (10.2) | 22 (46.8) | 12 (23.5) | <.0001 | .02 |
| Fetal/neonatal composite outcome (n), (%) | 13 (9.5) | 9 (19.2) | 9 (17.7) | .17 | .87 |
Maternal composite outcome was defined as any adverse maternal outcome of postpartum hemorrhage, predelivery platelet transfusion, peripartum transfusion of any blood product, hemoglobin reduction of 30 g/L. Fetal/neonatal composite outcome was defined as any adverse neonatal event, including stillbirth, preterm birth before 34 wk of gestation, small for gestational age size (birth weight below 10th percentile for gestational age), or Apgar score <7 at 5 min.
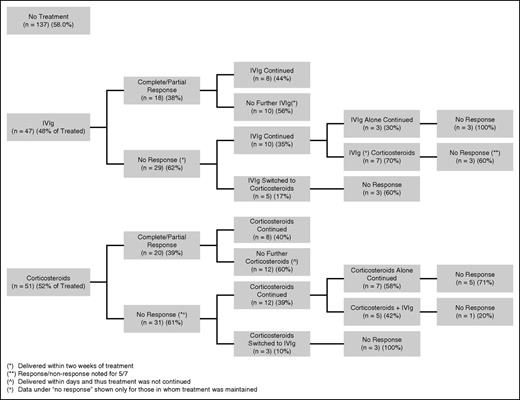
The response in platelet count according to treatment strategy is presented in Figure 2. The rise in platelet count was measured at a mean (SD) of 2 (±1) days following initiation of IVIg and 16 (±19) days following initiation of corticosteroids. Response occurred in 18/47 (38%) pregnancies treated initially with IVIg and 20/51 (39%) pregnancies treated initially with corticosteroids (P = .85). Two (2/4) responded to dexamethasone. In the majority of pregnancies, a lack of response in platelet count to the initial intervention persisted despite continuation of that agent, substitution for, or addition of the agent alternate to the one initially started. There was an 80% response in platelet count when IVIg was added after corticosteroids, compared with a 40% response when corticosteroids were added after IVIg.
Response rates according to treatment strategies for individuals in whom treatment with either agent was maintained.
Response rates according to treatment strategies for individuals in whom treatment with either agent was maintained.
There were no differences in neonatal outcomes between the 2 treated groups. The mean nadir in neonatal platelet counts from treated pregnancies compared with neonates from untreated pregnancies was 182 ± 104 × 109/L vs 205 ± 74 × 109/L, respectively; P = .24 and for pregnancies treated with IVIg compared with pregnancies treated with corticosteroids was 182 + 104 × 109/L vs 181 ± 104 × 109/L, respectively; P = .89.
A cranial ultrasound was completed in 25 neonates (45%) with platelet counts <150 × 109/L, and 2 neonates (8%) were diagnosed with intracranial hemorrhage. The mothers of these neonates had a history of ITP but maintained a platelet count that did not require treatment. One of the neonates had a nadir platelet count of 135 × 109/L and was delivered by emergency cesarean section due to placental abruption at 38 weeks of gestation; the other was born by spontaneous vaginal delivery at 38 weeks of gestation and reached a platelet count nadir of 18 × 109/L on day 3 postnatally, having had a cord platelet count of 186 × 109/L. Neonatal alloimmune thrombocytopenia was diagnosed in 3 thrombocytopenic neonates (5%) in this cohort, none of whom had ICH.
Post-hoc analysis
A history of splenectomy was noted in 21/235 (9%) pregnancies for which data were available, and in 16/136 (12%) of pregnancies that did not require IVIg or corticosteroids.
In pregnancies of splenectomized women, the mean maternal platelet count at the time of delivery was 243 × 109/L (±128), with a platelet count <100 × 109/L documented in 3 pregnancies. In women with a history of splenectomy, the mean maternal platelet count nadir was 197 × 109/L (±135), compared with 67 × 109/L (±48) in pregnancies without a history of splenectomy (P < .0001). Treatment was not required in 16/21 (76%) of splenectomized pregnancies, compared with 120/213 (56%) pregnancies without splenectomy (P = .07). Maternal platelet counts in the subgroup of untreated pregnancies ranged from 102 × 109/L to 544 × 109/L, with a mean (SD) of 280.6 (117.7) × 109/L.
The mean neonatal platelet count nadir in the 18 splenectomized pregnancies for which complete data were available was 189 × 109/L (±106), compared with 195 × 109/L (±88) in the 185 nonsplenectomized pregnancies (P = .71). Five neonates (28%) from pregnancies with a history of splenectomy were thrombocytopenic (< 150 × 109/L), as were 51 neonates (28%) from pregnancies without a maternal history of splenectomy. In the subgroup of pregnancies with a history of maternal splenectomy, neonatal thrombocytopenia was noted in 2/5 (40%) neonates whose mothers were treated in comparison with 13 neonates (23%) whose mothers did not require treatment.
Discussion
This retrospective study of 235 pregnancies in 195 women with ITP explored the effects of IVIg and corticosteroids on pregnancy outcomes. IVIg and corticosteroids were used with a similar frequency as the initial regimen in those who required treatment. There was no difference in maternal platelet counts at delivery between those treated with IVIg and those treated with corticosteroids as the initial agent. Response to treatment was observed in ∼ 40% of patients treated with either IVIg or corticosteroids. The sole difference between the treatments was a higher maternal composite outcome noted in the IVIg group. A trend toward fewer pregnancies in the corticosteroid group requiring peripartum transfusion of any blood product was also observed. Neonatal platelet nadir was reached as late as day 6 of life, following a normal cord platelet count.
In the nonpregnant population, corticosteroids are the first-line treatment of ITP and have been shown to induce an initial response in up to 85% of patients treated with high-dose dexamethasone15 and 60% to 67% treated with prednisone,16,17 although others have reported prednisone response rates as low as 40%.18,19 In contrast, response rates of 60% to 70% have been reported for IVIg.16,20 In the current study, less than half the pregnancies responded to the initial treatment, regardless of whether IVIg or corticosteroids (with doses of prednisone ∼1 mg/kg/d) were used.
The response to ITP therapies observed in the current study was lower than the response reported for the nonpregnant population. Although several studies describe treatment of ITP during pregnancy,21-23 they do not comment on response rates. Webert et al2 does address this parameter, citing a platelet count response in 11/20 (55%) IVIg-treated (55%) and 3/8 (38%) corticosteroid-treated pregnancies. Compared with that study, our observed response rate to IVIg was lower (18/47 [38%]), whereas our response rate to corticosteroids was comparable (20/51 [39%]). Our study has the advantage of a larger sample size and rigorous statistical analysis using GEE modeling to account for correlated data from repeat pregnancies of the same mother. Our observation of a relative resistance to ITP treatment during pregnancy requires further validation in prospective studies. We speculate that increased potency of antiplatelet antibodies during pregnancy, pregnancy-associated changes in platelet turnover,24 or altered drug metabolism may contribute to the lower response rates we observed.
The optimal dose of corticosteroids has not been determined. Although some studies have used higher prednisone doses of 50 mg/d, or 1 mg/kg/d,2,25 lower starting doses of prednisone (10 mg daily) have also been suggested for use in pregnancy.5 A large proportion of patients in our study were treated with prednisone doses in the higher range (median prednisone dose of 50 mg/d [IQR 35-75 mg/d], translating to 75% treated with doses of 0.5-1.0 mg/kg/d). Despite using a higher dose of corticosteroids, the response rate to corticosteroids in this study was lower than observed in nonpregnant patients.15,16 This finding may warrant consideration of starting corticosteroid therapy earlier in the third trimester in order to maximize the likelihood of reaching target platelet counts in time for delivery and raises the possibility that lower corticosteroid doses are ineffective in this setting.
Choice of a therapeutic agent sometimes depends on its inherent toxicities. In our study, adverse events were noted in 13% IVIg-treated pregnancies and in 13% of corticosteroid-treated pregnancies. Considering the retrospective nature of the study and reliance on medical record review, information on adverse events is likely underestimated.
Given the imperfect efficacy of corticosteroids and IVIg and given concerns over potential toxicities, exploration of novel ITP therapies is enticing. Rituximab, a monoclonal antibody against B-cell surface antigen CD20, is one such agent for which pregnancy data are accumulating.26 Another promising possibility comes in the form of Romiplostim, a thrombopoietin receptor agonist, the use of which has been documented in case reports, thus far without reported fetal complications.27 Nevertheless, more data on the short-term and long-term safety of these more novel therapies in pregnancy are undeniably needed before their role in this setting becomes clear.
In contrast to other studies, where 22% to 30% of pregnancies had moderate to severe bleeding,2,25 no critical maternal bleeding events occurred in the current study, and antenatal bleeding was secondary to obstetrical complications. This difference is likely due to the variable definitions of bleeding adopted by studies. For instance, although mucocutaneous bleeding was excluded from the definition of bleeding in the current study unless transfusion or hospitalization was required, it was part of the definition of bleeding in 2 previously published studies.2,25
Consistent with previous reports,25,28 there were no neonatal deaths in this study, and fetal/neonatal outcomes were overall favorable and comparable whether their mothers were treated with IVIg or corticosteroids. In particular, the risk of preterm birth postulated in some reports for pregnancies with maternal antenatal prednisone exposure29 was not apparent in our study, with no observed difference between the IVIg and corticosteroid groups in the rate of preterm birth below 37 or below 34 weeks’ gestation.
Severe neonatal thrombocytopenia and intracranial hemorrhage have been reported in the range of 4.6% to 29.9% and 0% to 3.7%, respectively.2,25,28,30 Our data are consistent with previous reports, in that severe thrombocytopenia occurred in 9% of all neonates, irrespective of maternal platelet counts, and 2 neonates experienced ICH. There were no other serious adverse effects observed, although nonsevere reactions may not have been well documented.
Although the nadir platelet count in the majority of thrombocytopenic neonates was reached by postnatal day 3, it was noted as late as day 6 of life. Of the 14 neonates in whom platelet count nadir was encountered at or beyond 3 days of life, 3 were premature, born at 35, 25, and 31 weeks’ gestation, respectively. Although prematurity is a risk factor for neonatal thrombocytopenia, this complication is generally encountered in the immediate newborn period, up to day 3 of life.31 Thus, prematurity as the sole cause of thrombocytopenia in these neonates is unlikely. Although delayed neonatal thrombocytopenia, appearing beyond 72 hours of life, is thought by some to be associated with sepsis and necrotizing enterocolitis,31 this has not been the case for the neonates in our study.
Also of significance is the finding that 9 neonates (11%) with normal cord platelet counts were found to have a reduction in their platelet count on repeat measurement. These findings highlight the need for determination of cord platelet counts in all neonates born to mothers with active or previous ITP and the need for continued monitoring of the neonatal platelet count during the first week of life, despite normal cord platelet counts. In addition to maternal placental transfer of antiplatelet immunoglobulin G, breastfeeding has been linked with prolonged neonatal thrombocytopenia.32 Milk from women who had ITP in pregnancy was found to have antiplatelet immunoglobulin A antibodies of varying levels.32 Neonatal thrombocytopenia of the index patient described by Hauschner et al resolved at 4 months, once breastfeeding was discontinued.32 Our study did not examine breastfeeding status and long-term neonatal platelets counts. However, future prospective studies examining this association would be worthwhile.
Akin to previous investigations,21,25,28,33 there was no correlation in our study between maternal platelet count at delivery and neonatal thrombocytopenia. However, history of previous pregnancy complicated by neonatal thrombocytopenia was identified as a risk factor for recurrence in subsequent pregnancies,21 suggesting that the effects of ITP on the neonate can be predicted by the clinical course of its siblings2,21 and hinting that ITP course and potency of maternal antibodies may persist from 1 pregnancy to the next.34 Accounting for such correlated data, permitted by GEE modeling, is imperative.
In line with a prior study demonstrating maternal splenectomy as a risk factor for neonatal thrombocytopenia requiring treatment,23 our study noted thrombocytopenia in 28% of neonates whose mothers were splenectomized. Furthermore, neonatal thrombocytopenia was noted in 40% of pregnancies with a history of maternal splenectomy where maternal treatment was required, in comparison with 23% of pregnancies with a history of splenectomy where maternal treatment was not needed. This observation is possibly suggestive of more active pathology in the group of women with splenectomies who required treatment, conceivably on the basis of continued antibody activity with potential to cross the placenta and affect neonatal platelets, although the paucity of cases in this subgroup precludes any definitive conclusions in this regard. Nevertheless, our observations suggest that monitoring of neonatal platelet counts is warranted in neonates of all women with a history of ITP, regardless of maternal platelet counts or the decision to institute treatment, and inclusive of splenectomized women whose platelet counts may have normalized.
No differences in the rate of cesarean section or operative vaginal delivery were observed between the treated and untreated groups. In context of current recommendations, it is worth noting that instrumental assistance to achieve vaginal birth was offered in 8.5%, 2.9%, and 2.3% of the untreated, IVIg-treated, and corticosteroid-treated groups, respectively. Nine of the neonates were born to mothers who did not require treatment of ITP during that particular pregnancy, and cord platelet counts were not available for 6 of them, whereas in one, the initial cord platelet count was 121 × 109/L with a nadir of 80 × 109/L on day 2 of life. This finding might potentially indicate false reassurance with respect to fetal/neonatal risk in the subset of patients with maternal ITP, where treatment is not required.
Although these numbers are too small to allow for conclusions to be drawn, the implications of more complex delivery in the setting of an unknown fetal platelet count merit discussion. The American College of Obstetricians and Gynecologists recommends against operative vaginal delivery (ie, forceps or vacuum assistance) in a fetus with a known or suspected bleeding disorder,35 acknowledging that these recommendations are based on limited and inconsistent scientific literature. The guidance stems from reported complication rates associated with instrumentation at the time of delivery.36 Although neonatal injury remains rare with the use of either instrument, bleeding events, such as cephalhematoma, have been found to occur with greater frequency (odds ratio 2.38, confidence interval 1.60-3.37) following vacuum extraction than forceps assistance.37 Studies examining the impact of operative vaginal delivery on outcomes of neonates with suspected bleeding disorders are lacking. Likewise, recommendations opposing assisted vaginal delivery in this setting must be balanced against the well-supported risks of maternal and neonatal morbidity of cesarean section in the second stage of labor,38 where the risk of possible trauma induced by the elevation of the impacted fetal head into the abdominal incision, often required to effect delivery, must also be considered. Further studies addressing these risks remain needed.
Our study is the first to compare the effectiveness of treatment with IVIg to treatment with corticosteroids in pregnancy. Strengths of this study were the statistical methodology, which accounted for dependency effects related to repeat pregnancies from the same mother, an approach that has not been employed in previous studies on this topic, and the large sample size with data capture from 2 centers, for a relatively rare disease. Study limitations include its retrospective design, necessitating reliance on the type and detail of information available in the medical record and precluding standardization of criteria for initiation of treatment or transfusion of blood products. The definition of gestational thrombocytopenia may have led to exclusion of a small number of pregnancies that in fact had ITP, as occasionally the distinction between gestational thrombocytopenia and ITP is unclear, in that occasionally platelet counts lower than 70 × 109/L are seen in those with gestational thrombocytopenia and at times platelet counts normalize postpartum in those with ITP. Information on adverse events may not have been adequately captured owing to reliance on information found in the medical record, which may have underrepresented more minor events. Our data did not allow for an analysis of hypertension, diabetes, or other pregnancy-related adverse outcomes, data of benefit to clinicians, given similar efficacy of IVIg and corticosteroids. Although the sample size of this study was large, event rates (maternal bleeding) were low; thus, inferences about treatment effects are somewhat limited.
This observational study comparing IVIg and corticosteroids in pregnant women with ITP demonstrated no significant differences between the 2 regimens, but highlighted the need for ongoing neonatal platelet count monitoring throughout the first week of life, regardless of the maternal platelet count. Prospective studies are needed to better characterize the safety of these regimens, to determine the optimal dose of corticosteroids, to identify risk factors for neonatal thrombocytopenia, and to explore new therapeutic options.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Canadian Blood Services Small Projects Fund and Canadian Institute of Health Research/Canadian Blood Services New Investigator Award (N.S.). P.S.S. is supported by an Applied Research Chair in Reproductive and Child Health Services from the Canadian Institutes of Health Research. D.M.A. is supported by the JG Kelton Chair in Translational Research from McMaster University.
Authorship
Contribution: D.S. contributed to the study concept, data collection, interpretation of the data, drafting of the manuscript, manuscript revision, and approval of the final version; N.S. contributed to the study concept, interpretation of the data, drafting of the manuscript, manuscript revision, and approval of the final version; X.Y.Y. contributed to the statistical analyses and interpretation of the data, writing of the statistical section of the manuscript, manuscript revision, and approval of the final version; S.G. contributed to the data collection, interpretation of the data, manuscript revision, and approval of the final version; B.D.F. contributed to the study concept, data collection, interpretation of the data, manuscript revision, and approval of the final version; D.M.A. contributed to the study concept, interpretation of the data, manuscript revision, and approval of the final version; P.S.S. contributed to the data analysis and interpretation, manuscript revision, and approval of the final version; and A.K.M. contributed to the study concept, data collection, analysis and interpretation of data, drafting of the manuscript, manuscript revision, and approval of the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dongmei Sun, Department of Medicine, London Health Sciences Centre, 800 Commissioners Rd E, Room E6 308, London, ON N6A 5W9, Canada; e-mail: dongmei.sun@lhsc.on.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal