In this issue of Blood, Wang et al describe a novel platform for improving our understanding of hematopoietic stem and progenitor cell (HSPC) behavior.1
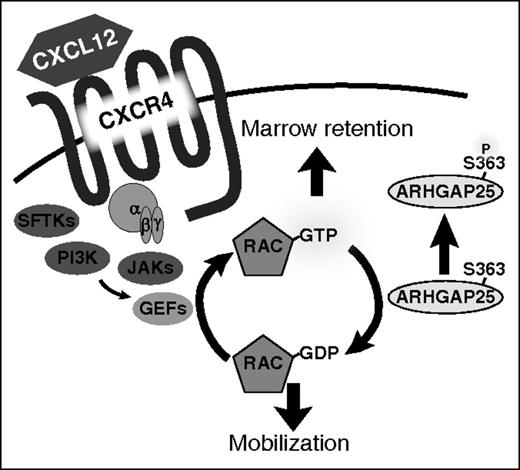
ARHGAP25 acts as a regulator of HSPC mobilization. Phosphorylation of serine 363 modulates ARHGAP25 activity and inhibits the ability of ARHGAP25 to suppress Rac-dependent downstream functions. Inhibition of ARHGAP25 activity leads to an augmentation of CXCL12 signaling and marrow retention. GDP, guanosine diphosphate; GEF, guanine nucleotide exchange factor; GTP, guanosine triphosphate; PI3K, phosphatidylinositol 3-kinase; SFTK, Src family tyrosine kinase. See Figure 5 in the article by Wang et al that begins on page 1465.
ARHGAP25 acts as a regulator of HSPC mobilization. Phosphorylation of serine 363 modulates ARHGAP25 activity and inhibits the ability of ARHGAP25 to suppress Rac-dependent downstream functions. Inhibition of ARHGAP25 activity leads to an augmentation of CXCL12 signaling and marrow retention. GDP, guanosine diphosphate; GEF, guanine nucleotide exchange factor; GTP, guanosine triphosphate; PI3K, phosphatidylinositol 3-kinase; SFTK, Src family tyrosine kinase. See Figure 5 in the article by Wang et al that begins on page 1465.
Although the process of HSPC mobilization has been used to benefit patients for close to 30 years, the mechanisms governing HSPC mobilization remain incompletely understood.2 This gap in knowledge has hampered the development of safer and more effective agents to mobilize HSPCs clinically. Mechanistic studies performed in genetically manipulated murine models over 15 years ago identified several key regulators of mobilization, most prominently the CXCL12/CXCR4 axis.3,4 Such studies led to the development (and ultimate US Food and Drug Administration [FDA] approval in 2008) of the CXCR4 antagonist plerixafor for the mobilization of CD34+ cells in patients with non-Hodgkin lymphoma and multiple myeloma (MM) prior to autologous HSPC transplantation.5,6 Since then, no new blood cell mobilizing agents have been approved and clinical development of such agents has slowed substantially. A more thorough understanding of cell migratory signaling pathways at the level of the intracellular protein may be needed to identify additional clinically exploitable targets for mobilization. To date, however, such studies in HSPCs have been hindered by the relative infrequency of these cells in the marrow microenvironment and peripheral blood. Combining multicolor flow cytometry and high-performance mass spectrometry, Wang and colleagues performed phosphoproteomic analyses of rare blood cell populations, namely HSPCs. They applied this platform to analyze the process of HSPC mobilization using a clinically relevant murine model. These studies are of significant interest not only for the information provided about differentially phosphorylated proteins comparing resting to mobilized HSPCs, but also as a method that could certainly be exploited to study the behavior of both normal and malignant stem cell populations, including leukemic stem cells, under a variety of conditions.
Of immediate relevance here, following the isolation of resting or mobilized murine Lin−Sca-1+c-Kit+ (LSK) cells via fluorescence-activated cell sorting, the LSK cells were then subjected to 3-dimensional reversed-phase liquid chromatography separation directly coupled to tandem mass spectrometry. They identified 178 differentially phosphorylated proteins of potential relevance to HSPC mobilization. They then focused on ARHGAP25, a GTPase activating protein (GAP) of the small Ras-related C3 botulinum toxin substrate 1 (Rac1). Rac1 is a member of the family of Rho GTPases, which exert influence on cell migration, actin remodeling, cell polarity, and HSPC mobilization.7 They validated ARHGAP25 as a potentially important regulator of HSPC mobilization whose function is modulated by phosphorylation. Deletion of ARHGAP25 in Arhgap25 knockout mice augmented HSPC chemotaxis to a CXCL12 gradient and hindered pharmacological mobilization, suggesting that it may serve as a novel target to enhance HSPC mobilization.
So why seek new insights into the mechanisms underlying HSPC trafficking? From the perspective of a clinician caring for patients undergoing autologous or allogeneic HSPC transplantation, one could argue that we already possess adequate means to procure HSPCs, either through simple bone marrow harvesting procedures or through stem cell mobilization techniques developed and used successfully over the past 30 years. Perhaps the agents we currently have at our disposal are good enough. But 10% to 15% of patients with lymphoma and MM do not mobilize HSPCs adequately, and these patients need better mobilizing strategies. Additionally, there are a number of important emerging clinical indications for HSPC mobilization where safer, more effective, and more rapid means to procure HSPCs would be highly desirable. Gene-therapy applications (eg, hemoglobinopathies, chronic granulomatous disease, etc) where larger quantities of hematopoietic stem cells (HSCs) available for gene transduction could improve persistence following transfer come to mind immediately, as do novel strategies combining HSPCs and other blood cells used to confer tolerance to solid organ transplants.8 Perhaps this platform could be used to monitor response to immunosuppressive therapy in patients with aplastic anemia. Even in the allogeneic blood cell transplant realm, where donors currently undergo painful bone marrow harvest or an inconvenient and transiently painful 5- to 6-day treatment with granulocyte colony-stimulating factor (G-CSF), there is a need for less toxic and more rapid HSPC mobilization techniques.
The platform described here by Wang and colleagues sought to identify novel regulatory pathways involved in HSPC mobilization (see figure). In addition to ARHGAP25, a number of additional candidates were discovered and await their further validation. However, there are several additional experiments not yet performed that could be envisaged. The authors studied the most common method used to procure HSPCs from peripheral blood of patients: cyclophosphamide followed by G-CSF. Although certainly clinically relevant, it would be of interest to determine whether there are differences in HSPC phosphoproteomic profile following other stimuli including agents blocking the CXCR4/CXCL12 interaction or antagonists of VLA-4/VCAM1, among other agents, particularly because some data suggest that although HSPC mobilization may be the final common end point, mobilization methods may differ substantially in the manner in which they affect the bone marrow microenvironment.9 The platform described could also be exploited to study HSC engraftment and HSC response to other environmental stimuli (eg, radiation) and, importantly, to better understand intracellular pathways in hematological malignancies such as acute myeloid leukemia, where persistence of drug-resistant leukemic stem cells following chemotherapy has been postulated to be a major source for treatment failure.10 So in this regard, the importance of this article lies not only in the unleashing of another pathway regulating HSPC mobilization, but also in demonstrating the potential of combining high-speed multicolor flow cytometry with high-throughput mass spectrometry for evaluating other rare cell populations under a variety of conditions.
The key to unlocking several mysteries of HSPC behavior may be within our grasp. We now have methods available to interrogate the molecular processes governing functions of rare cell populations during steady state and following extrinsic stimuli. The studies reported here by Wang and colleagues are hopefully just the beginning of the search for novel targets that will improve the lives of our patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal