Key Points
In thalassemia patients with cardiac siderosis, amlodipine combined with iron chelation resulted in more effective reduction of cardiac iron.
The combined treatment did not have any effect on serum ferritin and left ventricular ejection fraction.
Abstract
Cardiovascular disease resulting from iron accumulation is still a major cause of death in patients with thalassemia major (TM). Voltage-gated calcium-channel blockade prevents iron entry into cardiomyocytes and may provide an adjuvant treatment to chelation, reducing myocardial iron uptake. We evaluated whether addition of amlodipine to chelation strategies would reduce myocardial iron overload in TM patients compared with placebo. In a multicenter, double-blind, randomized, placebo-controlled trial, 62 patients were allocated to receive oral amlodipine 5 mg/day or placebo in addition to their current chelation regimen. The main outcome was change in myocardial iron concentration (MIC) determined by magnetic resonance imaging at 12 months, with patients stratified into reduction or prevention groups according to their initial T2* below or above the normal human threshold of 35 ms (MIC, 0.59 mg/g dry weight). At 12 months, patients in the reduction group receiving amlodipine (n = 15) had a significant decrease in MIC compared with patients receiving placebo (n = 15) with a median of −0.26 mg/g (95% confidence interval, −1.02 to −0.01) vs 0.01 mg/g (95% confidence interval, −0.13 to 0.23), P = .02. No significant changes were observed in the prevention group (treatment–effect interaction with P = .005). The same findings were observed in the subgroup of patients with T2* <20 ms. Amlodipine treatment did not cause any serious adverse events. Thus, in TM patients with cardiac siderosis, amlodipine combined with chelation therapy reduced cardiac iron more effectively than chelation therapy alone. Because this conclusion is based on subgroup analyses, it needs to be confirmed in ad hoc clinical trials. This trial was registered at www.clinicaltrials.gov identifier as #NCT01395199.
Introduction
Myocardial iron overload affects up to 50% of patients with thalassemia major (TM) in many parts of the world.1,2 Despite significant reductions in death rates because of early diagnosis and improved chelation treatment, iron cardiomyopathy is still responsible for a high proportion of deaths and hospitalization from arrhythmias and heart failure, especially in patients with high myocardial iron concentrations (MIC).3,4 Noninvasive assessment of MIC with magnetic resonance imaging (MRI) and early, more intensive chelation to reduce iron levels in the heart have been the main goal of most recent trials in this disease, with a corresponding reduction in the incidence of clinical events.5-8 Despite these advances, current options for treatment of myocardial iron overload are restricted to a limited number of chelation strategies, with important constraints from the need for elevated doses, concomitant side effects, heterogeneous access worldwide resulting from relatively high cost, or limited market availability.9
Once iron is taken up by cardiomyocytes, its removal from within these cells is slow. Myocardial clearance may take several years to occur even with very intensive chelation because of the specific mechanisms of iron handling in the heart.10,11 Experiments in mice suggest the possibility of preventing iron uptake by cardiomyocytes through voltage-gated calcium-channel blockade.12-15 Amlodipine is an inexpensive, widely available calcium-channel blocker with a well-known safety profile in both adults and children, and a first small, open-label study in humans showed its use reduced MIC as measured by MRI in TM patients.16 In this randomized trial, we sought to study whether the use of oral amlodipine in addition to standard iron chelation regimens in patients with TM can reduce MIC after 1 year of treatment.
Methods
Study design and participants
The study was designed as a multicenter, randomized, placebo-controlled, double-blind trial with an allocation rate of 1:1. Participants with TM were included if they were 6 years of age or older and had been receiving regular blood transfusions for at least 2 years (total lifetime red blood cell transfusions above 20 units). Exclusion criteria were a scheduled or already expected change in chelation strategy within the next 12 months (specifically, a change in chelator drug or change from monotherapy to combined therapy, for example); advanced clinical heart failure or ejection fraction below 35%; and formal contraindication for undergoing a MRI examination. The local ethics committees approved the study, and all participants (or their legal guardian) gave written informed consent. All authors had access to the primary clinical trial data.
Randomization and masking
Randomization was performed using a predefined computer-generated list without specific restrictions of blocks, which was kept by the pharmacy responsible for manufacturing the pills for the study (Formula & Cia, Campinas, Brazil). Allocation of patients and pill distribution were done by the central pharmacy, with the drug/placebo being shipped directly to the patient in tablets with identical appearance. Compliance was checked monthly during the outpatient/transfusion visits as well as through telephone contacts by a study technician. Concealment of the type of intervention was kept during the whole study for patients and health personnel involved in diagnosis, examination interpretation, and treatment.
Procedures
Patients were invited to participate during their routine outpatient appointments in 6 hematology centers in Brazil. Once selected, peripheral venous blood samples were collected for chemistry and hematology analyses and an MRI scan was performed if the patient had not undergone the examination within 30 days before enrollment in the trial. MRI scans were acquired according to a specific protocol to determine liver and myocardial T2* as well as left ventricular parameters in 1.5 T scanners following standardized techniques (details of the MRI protocols are described in the supplemental Data, available on the Blood Web site).17 Patients were stratified according to their initial MIC values: they were placed in the reduction group if baseline MIC was initially above the normal mean human threshold for iron concentrations (MIC >0.59 mg/g dry weight or T2*<35 ms)18,19 or the prevention group if MIC was below those values at baseline (MIC ≤0.59 mg/g or T2* ≥35 ms). After undergoing MRI scans, patients in each group were randomized to receive either oral placebo or amlodipine (5 mg/day for patients weighing more than 30 kg or 2.5 mg/day for patients weighing 30 kg and less). During the study, amlodipine/placebo doses could be lowered if the patient complained of adverse events commonly expected with the use of calcium-channel blockers. Changes in chelation therapy were allowed during follow-up at the primary physician’s discretion, especially in cases when patients had potentially dangerous levels of MIC or liver iron concentration (LIC). After 12 months, all patients repeated the MRI scan with the same parameters as the baseline examination. At 6 months, an additional MRI examination was also performed in a subgroup of patients that lived geographically close to 1 of the MRI centers. A central core laboratory concealed to treatment allocation and identity of the patient performed interpretation of the all MRI scans using a dedicated workstation (Circle Cardiovascular Imaging, Calgary, Canada; details in the supplemental data). MIC and LIC values were derived from myocardium and liver T2*, respectively, according to previously published reports.20,21 All MRI data were available to the primary physician throughout the study.
Outcomes
The primary outcome of the study was the change in MIC at 12 months from baseline in both arms (placebo and amlodipine) as defined by T2* values, with the comparison of effect of the treatment between the 2 subgroups based on subgroup–treatment effect interaction. This outcome was changed to evaluate change in MIC instead of T2* values after the publication of the correlation curves between myocardial T2* and MIC during the course of the trial because they showed a nonlinear correlation between these parameters with a more appropriate clinical use for MIC.20 The use of MIC allows for a more direct assessment of the linear changes in iron concentrations of the heart, as has been used in the liver in previous trials using LIC as the main outcome and not liver T2*.5,6,8 Secondary outcomes were change in MIC at 6 months, change in LIC at 12 months, serum ferritin, left ventricular ejection fraction, and incidence of adverse events at 6 and 12 months.
Statistical analysis
Sample size was defined based on previous pilot study data that showed a 27% reduction in myocardial iron in patients treated with amlodipine.16 For a power of 80% and α error of 0.05 to detect a similar difference in the primary outcome between groups assuming a 30% drop-out rate, the total number of patients for enrollment was calculated to be 62 for a final number of 43 patients to be analyzed (PASS 11, Kaysville, UT). The expected difference used in the power calculation was based on patients with initially high myocardial iron because patients with normal baseline MICs did not show significant follow-up changes in previous studies.22 We acknowledge that this might limit possible interpretations of our findings from underpowering of the subgroup analysis.
All data were analyzed on an intention to treat basis with a 2-tailed significance level of 5% (Medcalc Statistical Software, version 15.8, Ostend, Belgium). Baseline differences among the groups were compared using Student t test, χ2 test with Fisher’s exact test for proportions and Mann-Whitney in case of nonparametric variables (specifically, serum ferritin, myocardial T2*, MIC, and LIC). The respective changes in MIC, LIC, and serum ferritin were not normally distributed and were compared using Wilcoxon test. Subgroup–treatment effect interaction was analyzed using 2-way analysis of variance including treatment arm and subgroup classification as independent factors.23
Results
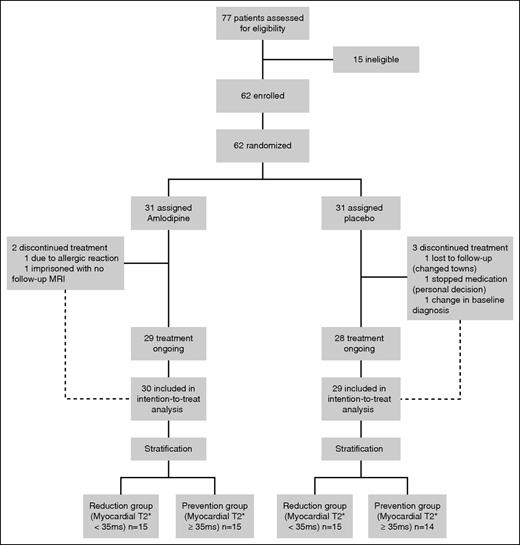
Seventy-seven patients were screened for the trial and 62 patients were randomized from October 1, 2011, to February 10, 2014 (Figure 1). The main reasons for exclusion during screening were prediction of chelation therapy change in the next 12 months and patients declining to participate. In the amlodipine arm, 1 patient was excluded from analysis from a lack of follow-up MRI scans; in the placebo arm, 1 patient was lost to follow-up and 1 patient was excluded because of review of the initial diagnosis from TM to hemoglobulin S/β thalassemia.
Baseline characteristics of all patients and in each of the treatment arms are shown in Table 1. No significant baseline differences were observed between the amlodipine and placebo arms. Baseline characteristics of the patients stratified into reduction and prevention groups are presented in supplemental Table 1, with no significant differences in treatment arms in these subgroups either. Cardiac iron overload defined by T2* <35 ms (MIC >0.59 mg/g) was observed in 50% of the amlodipine arm and 52% of the placebo arm, with 27% of the patients in the former arm with T2* <20 ms (MIC >1.16 mg/g) vs 14% in the latter (P = .33). No significant differences in initial MIC were found in the reduction group between patients allocated to amlodipine or placebo treatment, with a median of 1.31 mg/g (range, 0.64-12.81) vs 0.77 mg/g (range, 0.61-4.34), respectively (P = .17). There was 1 outlier in the amlodipine group whose removal brought the median in this group to 1.08 mg/g (range, 0.64-4.26). Because all other statistical results were similar after exclusion of this outlier, we chose to perform the analysis while keeping all patients.
Baseline characteristics of patients
| Characteristic . | Amlodipine (n = 30) . | Placebo (n = 29) . | All patients (n = 59) . |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 17 (57) | 12 (41) | 29 (49) |
| Age, y (range) | 23.3 ± 7.7 (12-38) | 23.5 ± 10.2 (8-49) | 23.4 ± 9.0 (8-49) |
| Weight, kg | 55.8 ± 11.4 | 55.9 ± 17.5 | 55.8 ± 14.6 |
| Body mass index, kg/m2 | 22.1 ± 3.3 | 22.3 ± 4.1 | 22.2 ± 3.7 |
| Hepatitis C, n (%) | 9 (30) | 6 (21) | 15 (25) |
| Splenectomy, n (%) | 5 (17) | 2 (7) | 7 (12) |
| Pretransfusional hemoglobin, g/dL | 9.4 ± 1.0 | 9.5 ± 1.0 | 9.5 ± 1.0 |
| Time since start of chelation therapy, y | 19.7 ± 6.7 | 20.3 ± 9.5 | 20.0 ± 8.1 |
| Chelation therapy at baseline, n (%) | |||
| DFO | 3 (10) | 4 (14) | 7 (12) |
| DFP | 5 (16) | 6 (21) | 11 (19) |
| DFX | 17 (57) | 13 (45) | 30 (51) |
| DFO + DFP | 5 (16) | 5 (17) | 10 (16) |
| DFO + DFX | 0 | 1 (3) | 1 (2) |
| Serum ferritin, ng/mL (median [range]) | 2638 (478-7282) | 1922 (386-13 113) | 2024 (386-13 113) |
| Myocardial T2*, ms (median [range]) | 34.1 (2.8-43.8) | 34.1 (6.8-42.7) | 34.1 (2.8-43.8) |
| Myocardial iron concentration, mg/g dry weight (median [range]) | 0.61 (0.45-12.81) | 0.61 (0.46-4.34) | 0.61 (0.45-12.81) |
| Left ventricular ejection fraction, % | 67.7 ± 5.5 | 67.1 ± 6.6 | 67.4 ± 6.0 |
| Myocardial T2* categories, n (%) | |||
| <10 ms | 4 (13) | 2 (7) | 6 (10) |
| 10 to ≤20 ms | 4 (13) | 2 (7) | 6 (10) |
| 20 to <35 ms | 7 (23) | 11 (38) | 18 (31) |
| ≤35 ms | 15 (50) | 14 (48) | 29 (49) |
| LIC, mg/g, median (range) | 11.3 (1.9-40.0) | 9.2 (1.5-33.3) | 9.6 (1.5-40.0) |
| LIC categories, n (%) | |||
| <7 mg/g | 10 (33) | 12 (42) | 22 (37) |
| 7 to ≤15 mg/g | 8 (27) | 9 (31) | 17 (29) |
| >15 mg/g | 12 (40) | 8 (28) | 20 (34) |
| Characteristic . | Amlodipine (n = 30) . | Placebo (n = 29) . | All patients (n = 59) . |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 17 (57) | 12 (41) | 29 (49) |
| Age, y (range) | 23.3 ± 7.7 (12-38) | 23.5 ± 10.2 (8-49) | 23.4 ± 9.0 (8-49) |
| Weight, kg | 55.8 ± 11.4 | 55.9 ± 17.5 | 55.8 ± 14.6 |
| Body mass index, kg/m2 | 22.1 ± 3.3 | 22.3 ± 4.1 | 22.2 ± 3.7 |
| Hepatitis C, n (%) | 9 (30) | 6 (21) | 15 (25) |
| Splenectomy, n (%) | 5 (17) | 2 (7) | 7 (12) |
| Pretransfusional hemoglobin, g/dL | 9.4 ± 1.0 | 9.5 ± 1.0 | 9.5 ± 1.0 |
| Time since start of chelation therapy, y | 19.7 ± 6.7 | 20.3 ± 9.5 | 20.0 ± 8.1 |
| Chelation therapy at baseline, n (%) | |||
| DFO | 3 (10) | 4 (14) | 7 (12) |
| DFP | 5 (16) | 6 (21) | 11 (19) |
| DFX | 17 (57) | 13 (45) | 30 (51) |
| DFO + DFP | 5 (16) | 5 (17) | 10 (16) |
| DFO + DFX | 0 | 1 (3) | 1 (2) |
| Serum ferritin, ng/mL (median [range]) | 2638 (478-7282) | 1922 (386-13 113) | 2024 (386-13 113) |
| Myocardial T2*, ms (median [range]) | 34.1 (2.8-43.8) | 34.1 (6.8-42.7) | 34.1 (2.8-43.8) |
| Myocardial iron concentration, mg/g dry weight (median [range]) | 0.61 (0.45-12.81) | 0.61 (0.46-4.34) | 0.61 (0.45-12.81) |
| Left ventricular ejection fraction, % | 67.7 ± 5.5 | 67.1 ± 6.6 | 67.4 ± 6.0 |
| Myocardial T2* categories, n (%) | |||
| <10 ms | 4 (13) | 2 (7) | 6 (10) |
| 10 to ≤20 ms | 4 (13) | 2 (7) | 6 (10) |
| 20 to <35 ms | 7 (23) | 11 (38) | 18 (31) |
| ≤35 ms | 15 (50) | 14 (48) | 29 (49) |
| LIC, mg/g, median (range) | 11.3 (1.9-40.0) | 9.2 (1.5-33.3) | 9.6 (1.5-40.0) |
| LIC categories, n (%) | |||
| <7 mg/g | 10 (33) | 12 (42) | 22 (37) |
| 7 to ≤15 mg/g | 8 (27) | 9 (31) | 17 (29) |
| >15 mg/g | 12 (40) | 8 (28) | 20 (34) |
Values expressed as mean ± standard deviation, unless otherwise specified.
DFO, deferoxamine; DFP, deferiprone; DFX, deferasirox.
Iron intake during the 12 months of follow-up was similar in both arms, with 173 ± 57 mg/kg per year in the amlodipine arm vs 177 ± 56 mg/kg per year in the placebo arm (P = .82). No significant differences were observed in modifications in chelation therapy between the 2 arms (P = .31): chelator doses were escalated by the primary physician in 30% of the patients in the amlodipine arm and in 24% in the placebo arm, and doses were decreased in 27% and 14% of the patients, respectively, with a full description of baseline and 12-month chelation regimens presented in supplemental Table 2.
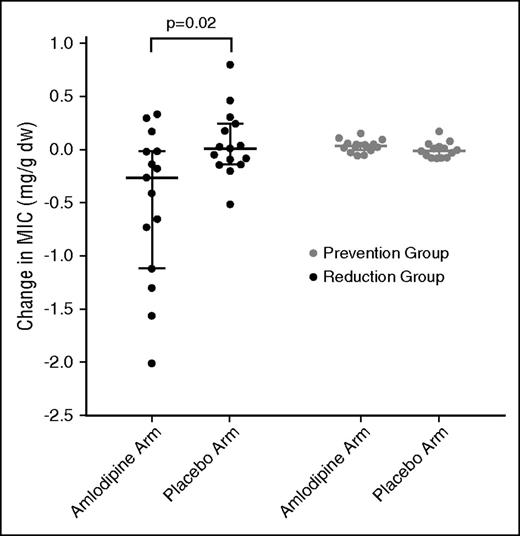
Between-subject treatment effects analysis provided strong evidence of a significant interaction between treatment effects and baseline MIC and indicated that the effect of treatment differed depending on the initial MIC (P = .005 for interaction; supplemental Figure 1). As a result, the effects of amlodipine were evaluated separately in each subgroup and not as a main effect in the whole cohort. Individual patient changes for all subjects are presented in supplemental Figure 2. Patients treated with amlodipine in the reduction group showed significant decrease in MIC at 12 months (primary outcome of the study) compared with patients treated with placebo after 12 months of treatment (Figure 2). MIC in the amlodipine arm significantly reduced from a baseline of 1.31 mg/g (range, 0.64-12.81) to 1.05 mg/g (range, 0.48-10.81), P = .02. MIC did not change significantly in patients receiving placebo, going from 0.77 mg/g (range, 0.61-4.34) at baseline to 0.75 (range, 0.49-4.59) at 12 months (P = .76). There was a significant difference in the median change in MIC in patients receiving amlodipine compared with placebo (median, −0.26 mg/g [95% confidence interval [CI], −1.02 to −0.01] vs 0.01 mg/g [95% CI, −0.13 to 0.23], P = .02; Table 2). The median percentage change in the amlodipine arm was −21.3% (95% CI, −31.9 to −1.33) vs +2.2% (95% CI, −15.5 to 8.5) in the placebo arm (P = .06). On a patient-by-patient evaluation, MIC increased in 8 patients (53%) in the placebo arm after 12 months compared with 3 patients (20%) that received amlodipine (relative risk, 0.38 [95% CI, 0.13–1.15], P = .09). There was no significant difference between the treatment arms in the number of patients that decreased or maintained their chelator doses: 9 (60%) in the amlodipine arm and 11 (73%) in the placebo arm.
Change in MIC in the placebo-treated patients vs amlodipine-treated patients in the reduction group and prevention group. Bars represent median and interquartile ranges; individual patients are represented by each circle. There was a significant change in MIC in the amlodipine arm in the reduction group (P = .02). dw, dry weight.
Change in MIC in the placebo-treated patients vs amlodipine-treated patients in the reduction group and prevention group. Bars represent median and interquartile ranges; individual patients are represented by each circle. There was a significant change in MIC in the amlodipine arm in the reduction group (P = .02). dw, dry weight.
Outcomes at 6 and 12 months
| Outcomes at 6 months . | Reduction group . | Prevention group . | ||||
|---|---|---|---|---|---|---|
| . | Amlodipine (n = 12) . | Placebo (n = 11) . | P . | Amlodipine (n = 12) . | Placebo (n = 12) . | P . |
| Change in MIC, mg/g | −0.18 (−0.95 to 0.03) | −0.06 (−0.12 to 0.12) | .16 | 0.0 (−0.05 to 0.08) | 0.05 (−0.08 to 0.10) | .73 |
| Change in MIC, % | −15.6 (−37.2 to 3.5) | −3.0 (−10.5 to 15.3) | .08 | 1.5 (−8.4 to 16.7) | 8.9 (−14.4 to 18.6) | .77 |
| Change in LIC, mg/g | −0.3 (−2.8 to 1.7) | 1.5 (−0.7 to 4.6) | .10 | 0.1 (−0.45 to 2.0) | 0.5 (−0.5 to 3.4) | .82 |
| Change in LIC, % | −4.6 (−17.3 to 17.1) | 29.2 (−7.8 to 56.5) | .046 | 3.2 (−5.8 to 22.1) | 8.1 (−7.6 to 29.8) | .91 |
| Change in SF, ng/mL | −451 (−1065 to 385) | 15 (−393 to 810) | .18 | −7 (−658 to 658) | 24 (−214 to 170) | .79 |
| Change in ejection fraction, % | −0.5 (−6.5 to 4.7) | 1.0 (−4.2 to 4.7) | .60 | 0 (−3.5 to 2.0) | 2.0 (0.18-5.8) | .21 |
| Outcomes at 6 months . | Reduction group . | Prevention group . | ||||
|---|---|---|---|---|---|---|
| . | Amlodipine (n = 12) . | Placebo (n = 11) . | P . | Amlodipine (n = 12) . | Placebo (n = 12) . | P . |
| Change in MIC, mg/g | −0.18 (−0.95 to 0.03) | −0.06 (−0.12 to 0.12) | .16 | 0.0 (−0.05 to 0.08) | 0.05 (−0.08 to 0.10) | .73 |
| Change in MIC, % | −15.6 (−37.2 to 3.5) | −3.0 (−10.5 to 15.3) | .08 | 1.5 (−8.4 to 16.7) | 8.9 (−14.4 to 18.6) | .77 |
| Change in LIC, mg/g | −0.3 (−2.8 to 1.7) | 1.5 (−0.7 to 4.6) | .10 | 0.1 (−0.45 to 2.0) | 0.5 (−0.5 to 3.4) | .82 |
| Change in LIC, % | −4.6 (−17.3 to 17.1) | 29.2 (−7.8 to 56.5) | .046 | 3.2 (−5.8 to 22.1) | 8.1 (−7.6 to 29.8) | .91 |
| Change in SF, ng/mL | −451 (−1065 to 385) | 15 (−393 to 810) | .18 | −7 (−658 to 658) | 24 (−214 to 170) | .79 |
| Change in ejection fraction, % | −0.5 (−6.5 to 4.7) | 1.0 (−4.2 to 4.7) | .60 | 0 (−3.5 to 2.0) | 2.0 (0.18-5.8) | .21 |
| Outcomes at 12 months . | Reduction group . | Prevention group . | ||||
|---|---|---|---|---|---|---|
| . | Amlodipine (n = 15) . | Placebo (n = 15) . | P . | Amlodipine (n = 15) . | Placebo (n = 14) . | P . |
| Change in MIC, mg/g | −0.26 (−1.02 to −0.01) | 0.01 (−0.13 to 0.23) | .02 | 0.04 (0-0.06) | −0.01 (−0.07 to 0.03) | .07 |
| Change in MIC, % | −21.3 (−31.9 to −1.33) | 2.2 (−15.5 to 8.5) | .06 | 8.9 (0.12-12.2) | −1.3 (−13.2 to 6.6) | .07 |
| Change in LIC, mg/g | 1.3 (−5.9 to 3.5) | 1.8 (1.2-6.0) | .16 | 1.0 (0.0-3.9) | 2.4 (−1.1 to 3.3) | .98 |
| Change in LIC, % | 23.1 (−32.2 to 45.0) | 47.8 (9.5-98.9) | .05 | 9.9 (0.7-47.8) | 27.7 (−8.3 to 42) | .95 |
| Change in SF, ng/mL | −515 (−1500 to 543) | 84 (−848 to 462) | .55 | 172 (−797 to 471) | 143 (−149 to 724) | .61 |
| Change in ejection fraction, % | 1.5 (−4.0 to 7.0) | 0 (−2.7 to 6.0) | .91 | 0 (−3.3 to 5.3) | −1.0 (−3.0 to 3.1) | .46 |
| Outcomes at 12 months . | Reduction group . | Prevention group . | ||||
|---|---|---|---|---|---|---|
| . | Amlodipine (n = 15) . | Placebo (n = 15) . | P . | Amlodipine (n = 15) . | Placebo (n = 14) . | P . |
| Change in MIC, mg/g | −0.26 (−1.02 to −0.01) | 0.01 (−0.13 to 0.23) | .02 | 0.04 (0-0.06) | −0.01 (−0.07 to 0.03) | .07 |
| Change in MIC, % | −21.3 (−31.9 to −1.33) | 2.2 (−15.5 to 8.5) | .06 | 8.9 (0.12-12.2) | −1.3 (−13.2 to 6.6) | .07 |
| Change in LIC, mg/g | 1.3 (−5.9 to 3.5) | 1.8 (1.2-6.0) | .16 | 1.0 (0.0-3.9) | 2.4 (−1.1 to 3.3) | .98 |
| Change in LIC, % | 23.1 (−32.2 to 45.0) | 47.8 (9.5-98.9) | .05 | 9.9 (0.7-47.8) | 27.7 (−8.3 to 42) | .95 |
| Change in SF, ng/mL | −515 (−1500 to 543) | 84 (−848 to 462) | .55 | 172 (−797 to 471) | 143 (−149 to 724) | .61 |
| Change in ejection fraction, % | 1.5 (−4.0 to 7.0) | 0 (−2.7 to 6.0) | .91 | 0 (−3.3 to 5.3) | −1.0 (−3.0 to 3.1) | .46 |
Values represent median (95% CI).
SF, serum ferritin.
A subgroup analysis including only patients presenting with a T2* <20 ms (MIC >1.16 mg/g) at baseline showed that patients treated with amlodipine had a significant median change in MIC (−0.92 mg/g: 95% CI, −1.43 to −0.53 vs 0.28 mg/g; 95% CI, −0.13 to 0.39 in the placebo arm, P = .02). Percent change in this subgroup was −26.8% (95% CI, −41.2 to −16.6) after 12 months of amlodipine vs +7.5% (95% CI, −8.9% to 23.0%) with placebo, P = .04. Paired individual MIC values at baseline and at 12 months stratified by cardiovascular event risk4 from patients in this subgroup are shown in Figure 3. No patient in the amlodipine arm with an initial T2* <20 ms displayed an increase in MIC after 12 months.
Baseline and 12-month MIC and myocardial T2* in the placebo (n = 4) and amlodipine arm (n = 8) in patients with a baseline T2* <20 ms (MIC > 1.16mg/g). The mild gray area represents patients at the lowest risk of developing heart failure, the light gray area represents mild to moderate risk, and the dark gray area represents MIC levels at which there is significant risk for cardiovascular complications.4 MIC and T2* can be converted using the formula [Fe] = 45.0 × (T2*)−1.22.20 A T2* of 10 ms represents a MIC of 2.71 mg/g, whereas a T2* of 20 ms corresponds to a MIC of 1.16 mg/g (data are presented in MIC [A] and T2* values [B]). No patient in the placebo group shifted from their original risk assessment, whereas 4 of 8 patients changed from severe to moderate risk or from moderate to low risk. All patients in the amlodipine arm reduced their baseline MIC values with a significant change at 12 months (2.9 mg/g [95% CI, 1.6-5.9] vs 2.1 [95% CI, 1.0-4.5], P = .008); in the placebo arm, no significant changes in MIC were observed (2.8 mg/g [95% CI, 1.7-3.8] vs 2.7 [95% CI, 1.7-4.1], P = .88).
Baseline and 12-month MIC and myocardial T2* in the placebo (n = 4) and amlodipine arm (n = 8) in patients with a baseline T2* <20 ms (MIC > 1.16mg/g). The mild gray area represents patients at the lowest risk of developing heart failure, the light gray area represents mild to moderate risk, and the dark gray area represents MIC levels at which there is significant risk for cardiovascular complications.4 MIC and T2* can be converted using the formula [Fe] = 45.0 × (T2*)−1.22.20 A T2* of 10 ms represents a MIC of 2.71 mg/g, whereas a T2* of 20 ms corresponds to a MIC of 1.16 mg/g (data are presented in MIC [A] and T2* values [B]). No patient in the placebo group shifted from their original risk assessment, whereas 4 of 8 patients changed from severe to moderate risk or from moderate to low risk. All patients in the amlodipine arm reduced their baseline MIC values with a significant change at 12 months (2.9 mg/g [95% CI, 1.6-5.9] vs 2.1 [95% CI, 1.0-4.5], P = .008); in the placebo arm, no significant changes in MIC were observed (2.8 mg/g [95% CI, 1.7-3.8] vs 2.7 [95% CI, 1.7-4.1], P = .88).
In the prevention group, no significant differences were observed between the amlodipine and placebo arms, with a change in MIC in the former of +0.04 mg/g (95% CI, 0-0.06) and −0.01 mg/g (95% CI, −0.07 to 0.03) in the latter, P = .07. The median baseline MIC of 0.54 mg/g went to 0.55 mg/g (range, 0.42-0.71 mg/g) with amlodipine and from 0.55 mg/g to 0.52 mg/g (range, 0.44-0.68 mg/g) with placebo. No patient treated in either arm in the prevention group developed a T2* <20 ms (MIC >1.16 mg/g), whereas 2/14 (14%) in the placebo group and 3/15 (20%) in the amlodipine group finished the study with a T2* <35 ms (MIC >0.59 mg/g). Chelator dose was increased in 3 patients in each group and decreased in 3 patients taking amlodipine.
Despite changes in MIC with amlodipine in the reduction group, no significant changes in ejection fraction were observed in these patients compared with patients treated with placebo (Table 2). Absolute and relative changes in LIC were not significant in any of the study arms. In the reduction group, in which patients significantly reduced MIC, no significant differences in final LIC were seen at 12 months (11.7 [95% CI, 5.5-22.7] for amlodipine vs 13.1 [95% CI, 4.9-22.5] for placebo, P = .98). There was no significant change in serum ferritin values across the groups by the end of the study.
At 6 months, a subgroup of patients underwent MRI scans to assess MIC and LIC. Although not reaching statistical significance, the amlodipine-treated arm in the reduction group showed changes in MIC when compared with the placebo arm at this time point (−0.18 vs −0.06 mg/g, respectively, P = .16). In patients with MIC >1.16 mg/g (n = 7), median MIC at 6 months was significantly lower than baseline values (2.1 mg/g vs 2.6 mg/g, median difference −0.76 [95% CI, −1.14 to .31] mg/g, P = .047) in patients receiving amlodipine, with no significant differences in MIC in the placebo arm. No significant changes were observed in ejection fraction, LIC, or serum ferritin at this time point.
No patient died or was admitted because of cardiovascular complications during the trial. Four mild adverse events were reported in the amlodipine arm vs none in the placebo arm (13% vs 0%, P = .11; supplemental Table 3). Three patients (10%) in the amlodipine arm had their initial dose of 5 mg/day reduced to 2.5 mg/day because of mild ankle edema (2 patients) and dizziness (1 patient). All events subsided with dose reduction. One patient developed a mild cutaneous allergic reaction and stopped the medication after 15 days of use while continuing in the study. No significant cases of hypotension or bradycardia occurred in either of the groups during follow-up.
Discussion
The results of this study show that oral amlodipine reduces myocardial iron overload when added to standard iron chelation in TM patients with myocardial siderosis, subject to some statistical limitations. Amlodipine has been used as an antihypertensive agent for several decades in both adults and children with a well-known safety profile, low cost, and wide availability globally.24,25 This may suggest that our findings could be broadly applicable even at locations with more limited resources or experience, where a higher proportion of patients with myocardial siderosis are geographically situated.2 Because patient inclusion in this trial occurred in multiple sites and with few limiting inclusion or exclusion criteria, we believe that our findings can be generalized to a large percentage of patients with TM.
We found that amlodipine lowered levels of myocardial iron but did not affect iron storage in the liver or serum ferritin, which is compatible with the mechanisms by which the drug blocks iron uptake.26 Most of the hepatic and serum ferritin-bound iron does not depend on active uptake by voltage-gated channels, so blocking calcium channels was not expected to affect liver or ferritin-bound iron kinetics. One important aspect of the drug is that because of its long half-life of approximately 50 hours, at steady-state there are relatively small variations in plasma concentrations between doses.27 Therefore, the pharmacokinetics of amlodipine makes it suitable for single dosing any time of the day, allowing for continuous blockade of channels and thus possible prevention of labile plasma iron entrance into cell at all times.
Although we did not specifically evaluate iron excretion, blockade of calcium channels appears to increase the iron transport within the kidney by prolonging the activity of divalent metal transporter-1 channels and would favor the use of amlodipine especially with longer follow-ups.15 Although this mechanism of iron excretion is acknowledged, the majority of iron excreted will always be substantially derived by chelation. The allowed changes in chelation strategies during the trial might also have affected the differences observed in iron concentration in each of the organs studied. However, there were no significant differences in chelator strategies in the beginning of the study or in the proportion of patients changing chelators in each arm during follow-up. Supplemental Table 2 shows that most of the changes observed were from minor dose adjustments of current drugs and not substantial changes including switching of drugs or combination vs monotherapy modifications. If biases had indeed occurred, they would also favor treatment with amlodipine, considering that fewer patients in the treatment arm of the reduction group ended the study receiving combination therapy vs the placebo arm, despite the improvement of MIC only in the former. A separate analysis per iron chelator type would also be very interesting, but we thought this would not be feasible in this study because of the acknowledged sample size.
The reduction of 21.3% in MIC observed in patients with initial iron concentrations above the normal mean value represents a decrease of −0.26 mg/g in myocardial iron in 1 year and confirms the findings from the previous human pilot trial that showed an increase of 30% in heart T2*.16 This result is also in line with the experimental data that demonstrated that voltage-gated calcium-channel blockade appears to be very effective in the heart, reducing 45% of the iron uptake in mouse myocardial cells.13 In patients with more significant myocardial siderosis using the more clinically common cutoff for myocardial iron overload (T2* <20 ms, MIC >1.16 mg/g), this reduction was −0.92 mg/g, a greater drop than previously observed in patients with comparable initial MIC levels treated exclusively with iron chelators, in whom changes have been reported in the range of −0.26 to −0.89 mg/g.22,28,29 Interestingly, comparable reductions in MIC after 1 year of treatment had only been shown with either high-dose combination iron chelation therapy or intravenous, continuous deferoxamine.7,10,29,30 In patients with baseline myocardial T2* <20 ms that received amlodipine, despite the significant MIC reduction observed, only 25% of patients received combination therapy (vs 50% in the placebo group) and none received intravenous treatment, again suggesting a lack of bias in the chelation regimens interfering with the results. The findings on this group, despite the limited number of patients, reinforce the results found in the larger reduction group.
Limitations of this trial include the short observation period and the relatively small number of patients included to assess whether the addition of oral amlodipine can decrease the incidence of cardiovascular events in this population. Although our sample size calculation was based on previous experimental and pilot studies, that stratification was done after randomization, no block randomization was performed, and each subgroup was analyzed separately may have led to underpowering and possibility of confounders. This design imperfection has to be taken into account when analyzing our results, although we tried to circumvent these limitations by demonstrating the treatment–effect interactions and performing adequate statistical treatment to the data despite the reduced numbers. Previous randomized controlled studies in this area are relatively few and the number of patients in these studies is in accordance with our sample size as well. Nevertheless, we believe that the results, presented along with previous studies, allow us to reach the conclusions regarding the benefits of amlodipine.
One important clinical limitation that might have been affected by the reduced sample size was the observation that amlodipine did not increase left ventricular ejection fraction even in the reduction group. The relatively low prevalence of reduced ejection fraction or severe myocardial siderosis upon trial enrollment, limiting the power of the study to assess these outcomes, might have led to this lack of improvement because patients already started the trial with relatively high ejection fraction values with small ranges for improvement. Despite that, previous studies have established MIC as a strong and consistent surrogate for clinical outcomes with even small reductions of MIC associated with improved clinical outcomes.31 Future trials with patients, preferably with a higher degree of myocardial overload or even with established heart failure, might help indicate whether amlodipine can also improve global ventricular function. Patients who might benefit more from this therapy are exactly included in this group, as suggested by our results in the group with a T2* <20 ms (MIC >1.16 mg/g).
In the prevention groups, although there was no change in MIC in these patients, our data cannot rule out the possibility that extended use of amlodipine might prevent myocardial iron accumulation with a longer observation period. Although a T2* of 20 ms has traditionally been used as a clinical cutoff for relative normality, in practice the normal mean 1.5 T T2* value in the heart is considered 35 ms (corresponding to a MIC of 0.59 mg/g).18,19 Our rationale for choosing 35 ms as the stratification cutoff in this study was based on these normal human levels and on the median values for this entire cohort of patients of 34.1 ms (95% CI, 30.9-36.4 ms). In agreement with that, a recent study showed that patients with apparently normal T2* from 20 ms to 35 ms could present with lower myocardial native T1 values, which are associated with higher iron deposits in the myocardium, while no patient with a T2* >35 ms had abnormal T1 numbers.32 Therefore, although there would still be an expected improvement from 20 ms to 35 ms because abnormal iron deposition seems to be present, no further increases in T2* should be anticipated after those values are reached—when iron concentrations are very close to the normal threshold and other factors interfere with T2* relaxation time. Preventing an increase in MIC in patients with these initially normal values would certainly be desirable, and future studies with the use of amlodipine in longer follow-ups might address that hypothesis. Finally, future studies may also help investigate whether amlodipine can prevent iron overload or help iron removal in endocrine organs that also absorb iron through voltage-gated channels, particularly considering the close association of cardiac siderosis with endocrine complications and the correlation of pancreas and MICs.11,33
In conclusion, the use of oral amlodipine in addition to standard chelation therapy can reduce myocardial iron more effectively than iron chelation alone in patients with TM and myocardial siderosis, and may be useful in patients with a cardiac T2* below 35 ms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful for the assistance of ABRASTA (Brazilian Thalassemia Association) for help with patient communication and support and acknowledge the work of nurse Suzamar Braga Cabral in assembling part of the clinical database for the study.
This study was supported by the governmental funding agency Fundacao de Amparo a Pesquisa do Estado de São Paulo and the Sultan Bin Khalifa Translational Research Scholarship.
Authorship
Contribution: J.L.F was the principal investigator for the trial; S.R.L., M.P.A.V., K.Y.F., A.P., O.R.C., F.F.C., and S.T.S. were involved in the design of the trial; L.A.B.F. was involved in the analysis of the magnetic resonance imaging data; S.R.L., M.P.A.V., K.Y.F., G.R.B., D.M.T., T.H., and D.A.M. were involved in data collection and patient follow-up; and all authors contributed to the interpretation of the results and review of the manuscript.
Conflict-of-interest disclosure: J.L.F. reports personal fees from Novartis AG and Sanofi Aventis and nonfinancial support from Siemens AG, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Juliano L. Fernandes, Jose Michel Kalaf Research Institute, Av Jose de Souza Campos 840, Campinas, SP 13092-123, Brazil; e-mail: jlaraf@terra.com.br.

![Figure 3. Baseline and 12-month MIC and myocardial T2* in the placebo (n = 4) and amlodipine arm (n = 8) in patients with a baseline T2* <20 ms (MIC > 1.16mg/g). The mild gray area represents patients at the lowest risk of developing heart failure, the light gray area represents mild to moderate risk, and the dark gray area represents MIC levels at which there is significant risk for cardiovascular complications.4 MIC and T2* can be converted using the formula [Fe] = 45.0 × (T2*)−1.22.20 A T2* of 10 ms represents a MIC of 2.71 mg/g, whereas a T2* of 20 ms corresponds to a MIC of 1.16 mg/g (data are presented in MIC [A] and T2* values [B]). No patient in the placebo group shifted from their original risk assessment, whereas 4 of 8 patients changed from severe to moderate risk or from moderate to low risk. All patients in the amlodipine arm reduced their baseline MIC values with a significant change at 12 months (2.9 mg/g [95% CI, 1.6-5.9] vs 2.1 [95% CI, 1.0-4.5], P = .008); in the placebo arm, no significant changes in MIC were observed (2.8 mg/g [95% CI, 1.7-3.8] vs 2.7 [95% CI, 1.7-4.1], P = .88).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/12/10.1182_blood-2016-06-721183/4/m_1555f3.jpeg?Expires=1769079924&Signature=g0qzooo4TEa~wN5qOfwTEaFGd0bawc94h-RS0Eo~YtEk1BzJidjCVCGtbmwgJm6vlKC98VAhHqNUTXYwdOxyJ5280tadSpHBtIcFHe3Hxreaeuy2pNgJlG3~7DQS0Z21Z32OCaibohXwpQcyDlSkPjaRD0yWliz08iOXs4TpHLhFIjhzzMW4eEpKCQa-mY9hgEySyZ-ud5A5vJwjO1wqc24bohLFge7CnWu~cCIu-YykzEcG5hlcwaHMmAX4Toqo2knzPndvCQ0fKlpSi8KoHFf0RsQtw2ByYdFkKN40FGwHJ1Kr7eGKmHef4xkV3gGFeDNRiwJiZ9SuEhuHCNpBcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal