Key Points
Daratumumab plus lenalidomide/dexamethasone elicited an overall response rate of 81% (63% very good partial response or better).
Adverse events were manageable and in accord with the individual toxicity profiles of daratumumab and lenalidomide/dexamethasone.
Abstract
Daratumumab, a human CD38 immunoglobulin G1 kappa (IgG1κ) monoclonal antibody, has activity as monotherapy in multiple myeloma (MM). This phase 1/2 study investigated daratumumab plus lenalidomide/dexamethasone in refractory and relapsed/refractory MM. Part 1 (dose escalation) evaluated 4 daratumumab doses plus lenalidomide (25 mg/day orally on days 1-21 of each cycle) and dexamethasone (40 mg/week). Part 2 (dose expansion) evaluated daratumumab at the recommended phase 2 dose (RP2D) plus lenalidomide/dexamethasone. Safety, efficacy, pharmacokinetics, immunogenicity, and accelerated daratumumab infusions were studied. In part 1 (13 patients), no dose-limiting toxicities were observed, and 16 mg/kg was selected as the R2PD. In part 2 (32 patients), median time since diagnosis was 3.2 years, with a median of 2 prior therapies (range, 1-3 prior therapies), including proteasome inhibitors (91%), alkylating agents (91%), autologous stem cell transplantation (78%), thalidomide (44%), and lenalidomide (34%); 22% of patients were refractory to the last line of therapy. Grade 3 to 4 adverse events (≥5%) included neutropenia, thrombocytopenia, and anemia. In part 2, infusion-related reactions (IRRs) occurred in 18 patients (56%); most were grade ≤2 (grade 3, 6.3%). IRRs predominantly occurred during first infusions and were more common during accelerated infusions. In part 2 (median follow-up of 15.6 months), overall response rate was 81%, with 8 stringent complete responses (25%), 3 complete responses (9%), and 9 very good partial responses (28%). Eighteen-month progression-free and overall survival rates were 72% (95% confidence interval, 51.7-85.0) and 90% (95% confidence interval, 73.1-96.8), respectively. Daratumumab plus lenalidomide/dexamethasone resulted in rapid, deep, durable responses. The combination was well tolerated and consistent with the safety profiles observed with lenalidomide/dexamethasone or daratumumab monotherapy. This trial was registered at www.clinicaltrials.gov as #NCT01615029.
Introduction
Multiple myeloma (MM) remains an incurable disease. Most patients relapse despite treatment with proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs), and additional treatment options are limited.1,2 Recently, new antimyeloma drugs have entered clinical practice, including daratumumab (anti-CD38) and elotuzumab (anti-SLAMF7).3
Daratumumab is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that binds to CD38-expressing cells with high affinity and induces tumor cell death through diverse immune-mediated actions, including complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and antibody-dependent cellular phagocytosis, as well as induction of apoptosis and modulation of CD38 enzymatic activities.4-6 CD38 is overexpressed on myeloma cells,7,8 making it a rational target for MM. In addition, subpopulations of immune suppressive cells with high CD38 expression, such as regulatory T cells, regulatory B cells, and myeloid-derived suppressor cells, are sensitive to daratumumab.9 Cytotoxic T-cell expansion and activation and increased T-cell clonality have been observed after daratumumab monotherapy treatment in patients with relapsed or refractory disease, indicating a possible immunomodulatory role for daratumumab.9 Daratumumab has been shown to have synergistic antitumor activity in combination with lenalidomide in vitro through the activation of effector cells.10 Interestingly, CD38 upregulation has been reported with IMiDs, including lenalidomide,11 which supports the rationale for combining these agents in MM.
In phase 1/2 studies, daratumumab monotherapy exhibited a favorable safety profile and induced durable responses that deepened over time in heavily pretreated relapsed and refractory MM patients.12-14 Daratumumab showed tolerable safety and efficacy in combination with established regimens in patients with MM.15 This phase 1/2 study assessed the safety and efficacy of daratumumab in combination with lenalidomide/dexamethasone in patients with relapsed or relapsed/refractory MM.
Patients and methods
Patients
In the dose-escalation phase (part 1), eligible patients had relapsed MM after 2 to 4 prior lines of therapy, were 18 years of age or older, had an Eastern Cooperative Oncology Group performance status of 2 or less, and had measurable levels of M-component (serum M-component ≥1.0 g/dL or urine M-component ≥200 mg/24-hour sample). In the dose-expansion cohort (part 2), patients had received at least 1 line of MM therapy, achieved a partial response (PR) or better to at least 1 regimen, and had documented evidence of progressive disease as defined by International Myeloma Working Group criteria on or after their last regimen.
Patients were excluded if they had previously received an allogeneic stem cell transplantation (SCT) at any time or autologous SCT within 12 weeks of the first infusion, or antimyeloma treatment, radiotherapy, or any experimental drug or therapy within 2 weeks of the first infusion. Patients with clinical signs of meningeal involvement of MM or who had experienced prior grade 3 or higher deep vein thrombosis or pulmonary embolism were ineligible. Severe chronic obstructive pulmonary disease (forced expiratory volume in 1 second < 60% of predicted normal) or persistent asthma were exclusionary.
Study design
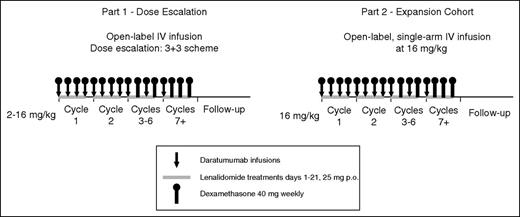
This phase 1/2, open-label, multicenter study was initiated on June 12, 2012 and, for this analysis, the clinical cutoff date was October 2, 2015. Part 1 was a standard 3+3 dose-escalation study (daratumumab 2, 4, 8, and 16 mg/kg), and part 2 was a dose-extension phase using the recommended phase 2 dose (RP2D; 16 mg/kg) determined in part 1 (Figure 1). For each dose-escalation cohort, an independent data monitoring committee evaluated aggregate safety data to approve escalation. The RP2D was determined on the basis of observed M-protein levels and was supported by pharmacokinetic data, part 1 safety profiles, and previous daratumumab experience in monotherapy studies.12,13 Ethics committees or institutional review boards at each site approved the study protocol and statistical analysis plan. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Written informed consent was provided by all patients.
Schematic overview of study design. Part 1, a dose-escalation phase, included 4 dose cohorts of daratumumab (2, 4, 8, and 16 mg/kg) in 28-day cycles in combination with lenalidomide 25 mg orally on days 1 through 21 and dexamethasone 40 mg once per week. Daratumumab was administered once per week during cycles 1 and 2, once every 2 weeks during cycles 3 through 6, and once every 4 weeks thereafter. In part 2, daratumumab 16 mg/kg was administered by using the same dosing schedule as in part 1 in combination with lenalidomide and dexamethasone. IV, intravenous; p.o., orally.
Schematic overview of study design. Part 1, a dose-escalation phase, included 4 dose cohorts of daratumumab (2, 4, 8, and 16 mg/kg) in 28-day cycles in combination with lenalidomide 25 mg orally on days 1 through 21 and dexamethasone 40 mg once per week. Daratumumab was administered once per week during cycles 1 and 2, once every 2 weeks during cycles 3 through 6, and once every 4 weeks thereafter. In part 2, daratumumab 16 mg/kg was administered by using the same dosing schedule as in part 1 in combination with lenalidomide and dexamethasone. IV, intravenous; p.o., orally.
Administration of study drugs
Each cycle was 28 days. Daratumumab was administered once per week for cycles 1 and 2, once every 2 weeks for cycles 3 through 6, and once every 4 weeks thereafter until disease progression or unacceptable toxicity. In part 1, a predose infusion of daratumumab (10% of the full dose but no more than 10 mg) was given the day before the first full infusion to minimize the risk of infusion-related reactions (IRRs). During part 2, one-third of patients received an accelerated first infusion of 500 mL over 3 hours (rather than 1000 mL over 6 hours) to evaluate safety and the incidence of IRRs during shortened infusions. Premedications included antihistamines, acetaminophen, and dexamethasone before each daratumumab infusion. Lenalidomide was administered orally at 25 mg/day on days 1 to 21 of each cycle; dexamethasone was administered at 40 mg/week, except when daratumumab was given (dexamethasone was administered at 20 mg pre– and post–daratumumab infusion). Additional details of drug administration are presented in the supplemental Data, available on the Blood Web site.
End points and assessments
The primary objective was to establish the safety profile of daratumumab in combination with lenalidomide/dexamethasone. Safety was assessed at each treatment visit by adverse event (AE) evaluation, clinical laboratory tests, physical examinations, vital signs, electrocardiograms, and Eastern Cooperative Oncology Group performance status. AEs were assessed by using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.16 Secondary objectives were to evaluate the efficacy of this combination, as well as the pharmacokinetics and immunogenicity of daratumumab.
Efficacy assessments included objective response rate (ORR; assessed by using a computerized algorithm based on International Myeloma Working Group Uniform Response Criteria for myeloma17 ), time to progression, response duration, and progression-free survival (PFS). A daratumumab-specific reflex immunofixation electrophoresis assay18 evaluated potential interference and confirmed complete responses (CRs) by distinguishing daratumumab from disease-related M-protein (supplemental Data).19 Cytogenetic analyses by fluorescence in situ hybridization were not readily available when the study was initiated.
Study oversight
The study design and analysis were planned by the investigators and sponsors, and data were collected by the investigators and their research teams. Final data analysis was conducted by Janssen Research & Development, which verified data accuracy.
Statistical analyses
No formal sample size determination was performed for part 1 because of the standard 3+3 dose-escalation design. For part 2, it was assumed that lenalidomide/dexamethasone would produce an ORR of 50% in the study population. With a two-sided α of 0.10 and assuming that the addition of daratumumab improves ORR to 75%, a sample size of 30 patients was selected to achieve 89% power to detect a difference. For efficacy, time-to-event end points, PFS, overall survival (OS), and response duration were analyzed by using Kaplan-Meier methodology.
Results
Patients and treatment
Thirteen patients were included in part 1 and were dosed in 4 daratumumab treatment groups: 2 mg/kg (n = 3), 4 mg/kg (n = 3), 8 mg/kg (n = 4), and 16 mg/kg (n = 3). Because of an administrative oversight, an extra patient received a dose of 8 mg/kg; this patient remained in the study. The median time since diagnosis was 3.8 years (range, 0.9-14.0 years). Patients received a median of 3 prior therapies (range, 2-4 prior therapies). All had received an IMiD (lenalidomide [76.9%] or thalidomide [53.8%]), and most patients received a PI and an IMiD (92.3%) or autologous SCT (69.2%). Additional baseline characteristics are summarized in Table 1. Median duration of treatment was 22.4 months (range, 2.8-33.4 months). At a median follow-up time of 23.5 months (range, 4.0-33.8 months), 6 patients (46.2%) had discontinued treatment, 4 (30.8%) because of disease progression and 2 (15.4%) because of an AE (cardiac disorder [at the 2 mg/kg dose] and neutropenia and thrombocytopenia [at the 8 mg/kg dose]), and 7 (53.8%) continued to receive treatment per protocol.
Demographic and baseline characteristics
| Characteristic . | Part 1 . | Part 2 . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daratumumab 2 mg/kg + lenalidomide/dexamethasone (n = 3) . | Daratumumab 4 mg/kg + lenalidomide/dexamethasone (n = 3) . | Daratumumab 8 mg/kg + lenalidomide/dexamethasone (n = 4) . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone (n = 3) . | Total (N = 13) . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone (N = 32) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Median age, y (range) | 69.0 (48-71) | 62.0 (61-65) | 56.0 (49-69) | 70.0 (56-76) | 62.0 (48-76) | 59.5 (41-76) | ||||||
| Sex | ||||||||||||
| Female | 0 | 33 | 0 | 67 | 23 | 31 | ||||||
| Male | 100 | 67 | 100 | 33 | 77 | 69 | ||||||
| ECOG score | ||||||||||||
| 0 | 2 | 66.7 | 2 | 66.7 | 1 | 25 | 3 | 100 | 8 | 61.5 | 19 | 59.4 |
| 1 | 1 | 33.3 | 1 | 33.3 | 3 | 75 | 0 | 0 | 5 | 38.5 | 12 | 37.5 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3.1 |
| No. of prior therapies | ||||||||||||
| Median (range) | 3.0 (3-4) | 2.0 (2-3) | 3.0 (3-4) | 3.0 (2-4) | 3.0 (2-4) | 2.0 (1-3) | ||||||
| ≥2 | 3 | 100 | 3 | 100 | 4 | 100 | 3 | 100 | 13 | 100 | 17 | 53.1 |
| Prior treatment | ||||||||||||
| IMiD | 3 | 100 | 3 | 100 | 4 | 100 | 3 | 100 | 13 | 100 | 23 | 71.9 |
| Lenalidomide | 3 | 100 | 3 | 100 | 3 | 75 | 1 | 33.3 | 10 | 76.9 | 11 | 34.4 |
| Thalidomide | 1 | 33.3 | 0 | 0 | 3 | 75 | 3 | 100 | 7 | 53.8 | 14 | 43.8 |
| PI | 3 | 100 | 2 | 66.7 | 4 | 100 | 3 | 100 | 12 | 92.3 | 29 | 90.6 |
| Bortezomib | 3 | 100 | 2 | 66.7 | 4 | 100 | 3 | 100 | 12 | 92.3 | 28 | 87.5 |
| PI + IMiD* | 3 | 100 | 2 | 66.7 | 4 | 100 | 3 | 100 | 12 | 92.3 | 21 | 65.6 |
| Bortezomib + lenalidomide* | 3 | 100 | 2 | 66.7 | 3 | 75 | 1 | 33.3 | 9 | 69.2 | 9 | 28.1 |
| Autologous stem cell transplantation | 1 | 33.3 | 2 | 66.7 | 4 | 100 | 2 | 66.7 | 9 | 69.2 | 25 | 78.1 |
| Prior chemotherapy† | 3 | 100 | 3 | 100 | 4 | 100 | 3 | 100 | 13 | 100 | 32 | 100 |
| Alkylating agents | 3 | 100 | 3 | 100 | 4 | 100 | 3 | 100 | 13 | 100 | 29 | 90.6 |
| Anthracyclines | 1 | 33.3 | 2 | 66.7 | 3 | 75 | 2 | 66.7 | 8 | 61.5 | 15 | 46.9 |
| Refractory to last line of therapy | 0 | 1 | 33.3 | 3 | 75 | 1 | 33.3 | 5 | 38.5 | 7 | 21.9 | |
| Refractory to therapy containing | ||||||||||||
| Lenalidomide | 0 | 1 | 33.3 | 2 | 50 | 1 | 33.3 | 4 | 30.8 | 1 | 3.1 | |
| Bortezomib | 0 | 2 | 66.7 | 3 | 75 | 1 | 33.3 | 6 | 46.2 | 5 | 15.6 | |
| Alkylating agents | 0 | 0 | 2 | 50 | 1 | 33.3 | 3 | 23.1 | 3 | 9.4 | ||
| PI only | 0 | 2 | 66.7 | 0 | 0 | 2 | 15.4 | 5 | 15.6 | |||
| IMiD only | 0 | 1 | 33.3 | 0 | 1 | 33.3 | 2 | 15.4 | 1 | 3.1 | ||
| Characteristic . | Part 1 . | Part 2 . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daratumumab 2 mg/kg + lenalidomide/dexamethasone (n = 3) . | Daratumumab 4 mg/kg + lenalidomide/dexamethasone (n = 3) . | Daratumumab 8 mg/kg + lenalidomide/dexamethasone (n = 4) . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone (n = 3) . | Total (N = 13) . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone (N = 32) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Median age, y (range) | 69.0 (48-71) | 62.0 (61-65) | 56.0 (49-69) | 70.0 (56-76) | 62.0 (48-76) | 59.5 (41-76) | ||||||
| Sex | ||||||||||||
| Female | 0 | 33 | 0 | 67 | 23 | 31 | ||||||
| Male | 100 | 67 | 100 | 33 | 77 | 69 | ||||||
| ECOG score | ||||||||||||
| 0 | 2 | 66.7 | 2 | 66.7 | 1 | 25 | 3 | 100 | 8 | 61.5 | 19 | 59.4 |
| 1 | 1 | 33.3 | 1 | 33.3 | 3 | 75 | 0 | 0 | 5 | 38.5 | 12 | 37.5 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3.1 |
| No. of prior therapies | ||||||||||||
| Median (range) | 3.0 (3-4) | 2.0 (2-3) | 3.0 (3-4) | 3.0 (2-4) | 3.0 (2-4) | 2.0 (1-3) | ||||||
| ≥2 | 3 | 100 | 3 | 100 | 4 | 100 | 3 | 100 | 13 | 100 | 17 | 53.1 |
| Prior treatment | ||||||||||||
| IMiD | 3 | 100 | 3 | 100 | 4 | 100 | 3 | 100 | 13 | 100 | 23 | 71.9 |
| Lenalidomide | 3 | 100 | 3 | 100 | 3 | 75 | 1 | 33.3 | 10 | 76.9 | 11 | 34.4 |
| Thalidomide | 1 | 33.3 | 0 | 0 | 3 | 75 | 3 | 100 | 7 | 53.8 | 14 | 43.8 |
| PI | 3 | 100 | 2 | 66.7 | 4 | 100 | 3 | 100 | 12 | 92.3 | 29 | 90.6 |
| Bortezomib | 3 | 100 | 2 | 66.7 | 4 | 100 | 3 | 100 | 12 | 92.3 | 28 | 87.5 |
| PI + IMiD* | 3 | 100 | 2 | 66.7 | 4 | 100 | 3 | 100 | 12 | 92.3 | 21 | 65.6 |
| Bortezomib + lenalidomide* | 3 | 100 | 2 | 66.7 | 3 | 75 | 1 | 33.3 | 9 | 69.2 | 9 | 28.1 |
| Autologous stem cell transplantation | 1 | 33.3 | 2 | 66.7 | 4 | 100 | 2 | 66.7 | 9 | 69.2 | 25 | 78.1 |
| Prior chemotherapy† | 3 | 100 | 3 | 100 | 4 | 100 | 3 | 100 | 13 | 100 | 32 | 100 |
| Alkylating agents | 3 | 100 | 3 | 100 | 4 | 100 | 3 | 100 | 13 | 100 | 29 | 90.6 |
| Anthracyclines | 1 | 33.3 | 2 | 66.7 | 3 | 75 | 2 | 66.7 | 8 | 61.5 | 15 | 46.9 |
| Refractory to last line of therapy | 0 | 1 | 33.3 | 3 | 75 | 1 | 33.3 | 5 | 38.5 | 7 | 21.9 | |
| Refractory to therapy containing | ||||||||||||
| Lenalidomide | 0 | 1 | 33.3 | 2 | 50 | 1 | 33.3 | 4 | 30.8 | 1 | 3.1 | |
| Bortezomib | 0 | 2 | 66.7 | 3 | 75 | 1 | 33.3 | 6 | 46.2 | 5 | 15.6 | |
| Alkylating agents | 0 | 0 | 2 | 50 | 1 | 33.3 | 3 | 23.1 | 3 | 9.4 | ||
| PI only | 0 | 2 | 66.7 | 0 | 0 | 2 | 15.4 | 5 | 15.6 | |||
| IMiD only | 0 | 1 | 33.3 | 0 | 1 | 33.3 | 2 | 15.4 | 1 | 3.1 | ||
ECOG, Eastern Cooperative Oncology Group.
Patients may have received these agents in different treatment regimens.
Includes either alkylating agents or autologous stem cell transplantation.
In part 2, 32 patients received daratumumab 16 mg/kg in combination with lenalidomide/dexamethasone (21 patients received standard first infusions and 11 received accelerated first infusions). Demographic and baseline characteristics are summarized in Table 1. Median time since initial diagnosis of MM was 3.2 years (range, 0.9-12.7 years); all patients received 3 or fewer lines of therapy, and median number of lines of therapy was 2 (range, 1-3 lines of therapy). Twenty-one patients (65.6%) received prior treatment with a PI and an IMiD (may have been in different treatment regimens), 11 (34.4%) received prior lenalidomide treatment (1 of whom was refractory), and 14 (43.8%) received thalidomide; 25 patients (78.1%) received prior autologous SCT.
In part 2, at a median follow-up of 15.6 months (range, 5.1-18.5 months), the median number of treatment cycles was 17 (range, 1-20 treatment cycles), and the median treatment duration was 14.8 months (range, 0.0-18.5 months). Patients received a median of 27 full infusions (range, 1-31 full infusions); median duration of the first infusion was 8.2 hours (range, 5.5-12.2 hours) for patients receiving a standard first infusion and 6.0 hours (range, 0.3-11.3 hours) for patients receiving an accelerated first infusion. The median duration of the second infusion was 6.5 hours (range, 3.8-7.7 hours) and 4.3 hours (range, 3.3-4.8 hours) in patients who received standard or accelerated first infusions, respectively, and the median duration of subsequent infusions was 3.9 hours (range, 0.8-8.7 hours) and 3.6 hours (3.1-6.0 hours), respectively.
Safety
In part 1, no dose-limiting toxicities were observed. Treatment-emergent AEs (TEAEs) were generally grade 1 or 2 in severity; the most common AEs (>25% of patients) are summarized in supplemental Table 1. Two patients received whole blood transfusions because of anemia; no TEAEs of hemolysis or transfusion reaction were reported. The most common IRRs (occurring in 2 or more patients) were allergic rhinitis and nasal congestion, and all were grade 1 or 2 (supplemental Table 2). Based on part 1 safety data, in which no dose-limiting toxicities were observed, and based on efficacy, safety, and pharmacokinetic results from monotherapy studies,12,13,20 daratumumab 16 mg/kg was chosen as the RP2D.
In part 2, the most common TEAEs (≥25%, all grades) were neutropenia (84.4%); cough (50.0%); diarrhea and muscle spasms (43.8% each); fatigue (34.4%); pyrexia and thrombocytopenia (31.3% each); nausea and hypertension (28.1% each); and upper respiratory tract infection, peripheral edema, and anemia (25.0% each; Table 2). Among 3 patients who received blood transfusions, no TEAEs of hemolysis or transfusion reaction were reported. Neutropenia, the most frequently reported (78.1%) grade 3 or 4 TEAE, was managed with growth factor treatment and/or lenalidomide dose modification. All neutropenia events were considered by the investigators as lenalidomide-related, and 53.1% of patients reported a TEAE of neutropenia that was also considered to be possibly daratumumab-related. There was one grade 3 TEAE of febrile neutropenia. In part 2, grade 3 or 4 infections/infestations occurred in 15.6% of patients and included upper respiratory tract infection, bronchitis, gastroenteritis, pneumonia, and viral pneumonia (1 patient each [3.1%]).
Summary of most common (≥25%) AEs (part 2)
| Event . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone (N = 32) . | |||
|---|---|---|---|---|
| All grades . | Grade ≥3 . | |||
| No. . | % . | No. . | % . | |
| Neutropenia | 27 | 84.4 | 25 | 78.1 |
| Cough | 16 | 50 | 0 | 0 |
| Diarrhea | 14 | 43.8 | 1 | 3.1 |
| Muscle spasms | 14 | 43.8 | 0 | 0 |
| Fatigue | 11 | 34.4 | 0 | 0 |
| Thrombocytopenia | 10 | 31.3 | 4 | 12.5 |
| Pyrexia | 10 | 31.3 | 0 | 0 |
| Hypertension | 9 | 28.1 | 3 | 9.4 |
| Nausea | 9 | 28.1 | 0 | 0 |
| Anemia | 8 | 25 | 4 | 12.5 |
| Upper respiratory tract infection | 8 | 25 | 1 | 3.1 |
| Peripheral edema | 8 | 25 | 0 | 0 |
| Event . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone (N = 32) . | |||
|---|---|---|---|---|
| All grades . | Grade ≥3 . | |||
| No. . | % . | No. . | % . | |
| Neutropenia | 27 | 84.4 | 25 | 78.1 |
| Cough | 16 | 50 | 0 | 0 |
| Diarrhea | 14 | 43.8 | 1 | 3.1 |
| Muscle spasms | 14 | 43.8 | 0 | 0 |
| Fatigue | 11 | 34.4 | 0 | 0 |
| Thrombocytopenia | 10 | 31.3 | 4 | 12.5 |
| Pyrexia | 10 | 31.3 | 0 | 0 |
| Hypertension | 9 | 28.1 | 3 | 9.4 |
| Nausea | 9 | 28.1 | 0 | 0 |
| Anemia | 8 | 25 | 4 | 12.5 |
| Upper respiratory tract infection | 8 | 25 | 1 | 3.1 |
| Peripheral edema | 8 | 25 | 0 | 0 |
Lenalidomide doses were reduced in 18 patients (56.3%) because of myelosuppression (40.6%); muscle cramps (6.3%); renal impairment, thromboembolic events, liver enzyme elevation, and fatigue (3.1% each); and other TEAEs (9.4%; including 1 patient with asthenia, constipation, and shaking and 1 patient with pulmonary embolism).
Three patients (9.4%) had TEAEs that led to discontinuation of all study treatments, with 1 case each of gastric adenocarcinoma (unrelated), viral pneumonia (related to all study drugs), and laryngeal edema (daratumumab-related in the accelerated infusion cohort). Three deaths were reported in part 2, with 2 deaths as a result of progressive disease and 1 as a result of viral pneumonia.
In part 2, a total of 18 patients (56.3%) experienced IRRs; all patients with IRRs experienced them during the first infusion, with none in the second infusion, and 3 (9.7%) during subsequent infusions. Events in more than 1 patient included cough (25.0%), allergic rhinitis (9.4%), nausea (9.4%), vomiting (9.4%), dyspnea (6.3%), and nasal congestion (6.3%; Table 3). The majority of IRRs were grade 1 or 2; only 2 patients had grade 3 IRRs (laryngeal edema and hypertension), and no grade 4 IRRs were reported. IRRs were more frequent during the accelerated first infusion schedule compared with standard infusions (any grade: 72.7% vs 47.6%; grade 3: 9.1% vs 4.8%; Table 3). All patients recovered and resumed subsequent cycles of treatment, except for the patient with laryngeal edema who discontinued treatment.
Summary of IRRs occurring in ≥2 patients (part 2)
| Event . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard infusion (n = 21) . | Shortened infusion (n = 11) . | Total (N = 32) . | ||||||||||
| All grades . | Grade 3 . | All grades . | Grade 3 . | All grades . | Grade 3 . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Any IRR | 10 | 47.6 | 1 | 4.8 | 8 | 72.7 | 1 | 9.1 | 18 | 56.3 | 2 | 6.3 |
| Cough | 3 | 14.3 | 0 | 0 | 5 | 45.5 | 0 | 0 | 8 | 25 | 0 | 0 |
| Allergic rhinitis | 1 | 4.8 | 0 | 0 | 2 | 18.2 | 0 | 0 | 3 | 9.4 | 0 | 0 |
| Nausea | 2 | 9.5 | 0 | 0 | 1 | 9.1 | 0 | 0 | 3 | 9.4 | 0 | 0 |
| Vomiting | 2 | 9.5 | 0 | 0 | 1 | 9.1 | 0 | 0 | 3 | 9.4 | 0 | 0 |
| Dyspnea | 2 | 9.5 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 6.3 | 0 | 0 |
| Nasal congestion | 2 | 9.5 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 6.3 | 0 | 0 |
| Event . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard infusion (n = 21) . | Shortened infusion (n = 11) . | Total (N = 32) . | ||||||||||
| All grades . | Grade 3 . | All grades . | Grade 3 . | All grades . | Grade 3 . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Any IRR | 10 | 47.6 | 1 | 4.8 | 8 | 72.7 | 1 | 9.1 | 18 | 56.3 | 2 | 6.3 |
| Cough | 3 | 14.3 | 0 | 0 | 5 | 45.5 | 0 | 0 | 8 | 25 | 0 | 0 |
| Allergic rhinitis | 1 | 4.8 | 0 | 0 | 2 | 18.2 | 0 | 0 | 3 | 9.4 | 0 | 0 |
| Nausea | 2 | 9.5 | 0 | 0 | 1 | 9.1 | 0 | 0 | 3 | 9.4 | 0 | 0 |
| Vomiting | 2 | 9.5 | 0 | 0 | 1 | 9.1 | 0 | 0 | 3 | 9.4 | 0 | 0 |
| Dyspnea | 2 | 9.5 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 6.3 | 0 | 0 |
| Nasal congestion | 2 | 9.5 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 6.3 | 0 | 0 |
Efficacy
In part 1, the ORR was 84.6%, with 5 (38.5%) stringent CRs (sCRs), 4 (30.8%) very good PRs (VGPRs), and 2 (15.4%) PRs (Table 4). Of the 4 lenalidomide-refractory patients, 2 achieved a VGPR and 1 achieved a PR.
Summary of responses
| Response . | Part 1 . | Part 2 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daratumumab 2 mg/kg + lenalidomide/ dexamethasone (n = 3) . | Daratumumab 4 mg/kg + lenalidomide/ dexamethasone (n = 3) . | Daratumumab 8 mg/kg + lenalidomide/ dexamethasone (n = 4) . | Daratumumab 16 mg/kg + lenalidomide/ dexamethasone (n = 3) . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone (n = 32) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | 95% CI . | |
| sCR | 1 | 33.3 | 2 | 66.7 | 2 | 50 | 0 | 8 | 25 | 11.5-43.4 | |
| CR | 0 | 0 | 0 | 0 | 3 | 9.4 | 2.0-25.0 | ||||
| VGPR | 1 | 33.3 | 1 | 33.3 | 0 | 2 | 66.7 | 9 | 28.1 | 13.7-46.7 | |
| VGPR or better* | 2 | 66.7 | 3 | 100 | 2 | 50 | 2 | 66.7 | 20 | 62.5 | 43.7-78.9 |
| PR | 1 | 33.3 | 0 | 1 | 25.0 | 0 | 6 | 18.8 | 7.2-36.4 | ||
| ORR† | 3 | 100 | 3 | 100 | 3 | 75 | 2 | 66.7 | 26 | 81.3 | 63.6-92.8 |
| MR | 0 | 0 | 1 | 25 | 0 | 2 | 6.3 | 0.8-20.8 | |||
| Stable disease | 0 | 0 | 0 | 1 | 33.3 | 3 | 9.4 | 2.0-25.0 | |||
| Progressive disease | 0 | 0 | 0 | 0 | 0 | — | |||||
| Not evaluable | 0 | 0 | 0 | 0 | 1 | 3.1 | 0.1-16.2 | ||||
| Response . | Part 1 . | Part 2 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daratumumab 2 mg/kg + lenalidomide/ dexamethasone (n = 3) . | Daratumumab 4 mg/kg + lenalidomide/ dexamethasone (n = 3) . | Daratumumab 8 mg/kg + lenalidomide/ dexamethasone (n = 4) . | Daratumumab 16 mg/kg + lenalidomide/ dexamethasone (n = 3) . | Daratumumab 16 mg/kg + lenalidomide/dexamethasone (n = 32) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | 95% CI . | |
| sCR | 1 | 33.3 | 2 | 66.7 | 2 | 50 | 0 | 8 | 25 | 11.5-43.4 | |
| CR | 0 | 0 | 0 | 0 | 3 | 9.4 | 2.0-25.0 | ||||
| VGPR | 1 | 33.3 | 1 | 33.3 | 0 | 2 | 66.7 | 9 | 28.1 | 13.7-46.7 | |
| VGPR or better* | 2 | 66.7 | 3 | 100 | 2 | 50 | 2 | 66.7 | 20 | 62.5 | 43.7-78.9 |
| PR | 1 | 33.3 | 0 | 1 | 25.0 | 0 | 6 | 18.8 | 7.2-36.4 | ||
| ORR† | 3 | 100 | 3 | 100 | 3 | 75 | 2 | 66.7 | 26 | 81.3 | 63.6-92.8 |
| MR | 0 | 0 | 1 | 25 | 0 | 2 | 6.3 | 0.8-20.8 | |||
| Stable disease | 0 | 0 | 0 | 1 | 33.3 | 3 | 9.4 | 2.0-25.0 | |||
| Progressive disease | 0 | 0 | 0 | 0 | 0 | — | |||||
| Not evaluable | 0 | 0 | 0 | 0 | 1 | 3.1 | 0.1-16.2 | ||||
Response was assessed by a computerized algorithm based on International Uniform Response Criteria Consensus Recommendations. Among the 11 patients with sCR/CRs, 3 were initially classified as having VGPR per International Myeloma Working Group criteria and then confirmed as having sCR/CR by daratumumab interference reflex assay.
MR, minimal response.
sCR + CR + VGPR.
sCR + CR + VGPR + PR.
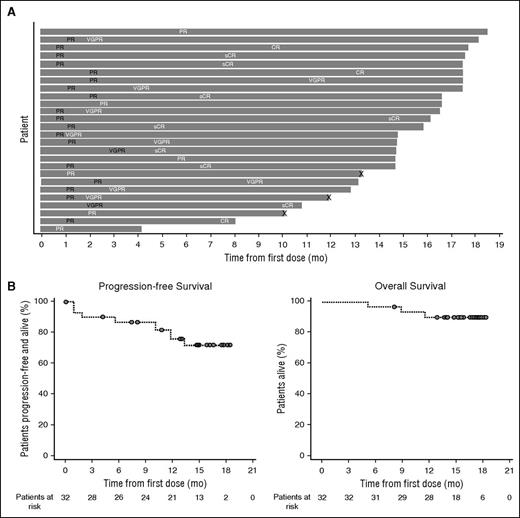
In part 2, the ORR was 81.3%, with 8 (25.0%; including 1 lenalidomide-refractory patient) sCRs, 3 (9.4%) CRs, 9 (28.1%) VGPRs, and 6 (18.8%) PRs (Table 4). Responses in 8 of the 11 patients with persistent IgG1κ immune fixation positivity were confirmed as CRs by using the immunofixation electrophoresis reflex assay (supplemental Data). In addition, 2 patients (6.3%) had a minimal response, resulting in a clinical benefit rate of 87.5%. Three patients (9.4%) had stable disease, and 1 patient was not evaluable (withdrew during the first cycle because of an IRR [laryngeal edema]). Responses were durable and tended to deepen over time. The median time to first and best response was 1.0 month (range, 0.5-5.6 months) and 5.1 months (range, 0.5-14.4 months), respectively. At 12 months, 91.0% (95% confidence interval [CI], 68.6-97.7) of responders had progression-free disease. At a median follow-up of 15.6 months (range, 5.1-18.5 months), the median duration of response was not reached. The timing and depth of response in each patient who achieved PR or better are illustrated in Figure 2A. Median PFS and OS were not reached at the time of this analysis (Figure 2B). The 18-month PFS and OS rates were 72.1% (95% CI, 51.7-85.0) and 90.4% (95% CI, 73.1-96.8), respectively. Twenty-two patients (68.8%) in part 2 continued to receive treatment at a median duration of treatment of 14.8 months (range, 0.0-18.5 months).
Duration of response, PFS, and OS in patients treated with daratumumab plus lenalidomide and dexamethasone in part 2. (A) Swim-lane plot of responders. Responses in black font indicate the first response, and those in white font indicate the best response. “X” indicates disease progression. (B) Kaplan-Meier curve of PFS and OS after daratumumab 16 mg/kg in combination with lenalidomide/dexamethasone in patients with relapsed or relapsed and refractory MM.
Duration of response, PFS, and OS in patients treated with daratumumab plus lenalidomide and dexamethasone in part 2. (A) Swim-lane plot of responders. Responses in black font indicate the first response, and those in white font indicate the best response. “X” indicates disease progression. (B) Kaplan-Meier curve of PFS and OS after daratumumab 16 mg/kg in combination with lenalidomide/dexamethasone in patients with relapsed or relapsed and refractory MM.
Pharmacokinetics and immunogenicity
All patients in parts 1 and 2 were included in the pharmacokinetics-evaluable population. Pharmacokinetics were consistent with daratumumab monotherapy.12,13 Mean maximum serum concentration (Cmax ) after the first dose of daratumumab 16 mg/kg was similar between part 1 (295.1 µg/mL) and part 2 (265.1 µg/mL). Daratumumab concentrations appeared to accumulate throughout the first 2 cycles of once-per-week dosing, after which concentrations began to decrease slightly with less frequent daratumumab administration (once every 2 weeks and once every 4 weeks). The mean trough concentration at the end of once-per-week dosing was 546 μg/mL (standard deviation, 226.3 μg/mL), and mean trough concentrations were maintained above 300 µg/mL throughout once-every-2-weeks dosing (supplemental Figure 1). No antidaratumumab antibodies were detected during parts 1 and 2 (in 13 and 24 evaluable patients, respectively).
Discussion
Daratumumab in combination with lenalidomide/dexamethasone showed remarkable efficacy in patients with relapsed or relapsed/refractory MM. The combination was well tolerated and consistent with the known safety profile for either lenalidomide/dexamethasone or daratumumab monotherapy. Moreover, patients receiving the RP2D of daratumumab (16 mg/kg) in part 2 had rapid, durable, and deepening responses. The 18-month PFS rate was 72% and ORR was 81% (34% CR or better and 62% VGPR or better). In most responders, response to treatment improved over time. Patients who achieved CR/sCR had initial responses of PR or VGPR, and several patients achieved a best response after 6 months of treatment. Remarkably, no further progression events have been reported during the last 6 months of the study. Interestingly, in part 2, 1 patient was lenalidomide-refractory and achieved an sCR that was ongoing at the time of data cutoff. It is likely that the trend of deepening responses contributed to the notable number of sCRs (25%) in this small study population. These data compare very favorably with phase 3 combination studies of lenalidomide/dexamethasone, including a pooled analysis of the lenalidomide/dexamethasone arms of pivotal phase 3 studies (61% ORR; 15% CR; median PFS, 11.1 months).21
Overall, the addition of daratumumab to lenalidomide/dexamethasone showed a manageable safety profile. In part 2, neutropenia, a known toxicity of lenalidomide, was the most common TEAE (84%, with 78% grade ≥3). Neutropenia was managed with treatment interruptions, dose reduction of lenalidomide, and growth factor administration; no treatment discontinuations occurred as a result of neutropenia. Only 1 case of febrile neutropenia was reported, and there were no cases of neutropenic sepsis, suggesting that, although the incidence of neutropenia was higher than historical data for the backbone regimen (grade 3 or 4 neutropenia rates of 25%-35%),22,23 this may not translate to more severe events. Despite the rate of neutropenia, grade 3 or 4 infections occurred in only 16% of patients. The number of patients who required dose reductions because of TEAEs (56%) was lower than that observed previously with lenalidomide/dexamethasone alone (∼76%),22,23 as was the rate of discontinuation because of TEAEs (9% vs 20%, respectively).22,23 The majority of IRRs were grade 1 or 2; only 2 patients had grade 3 IRRs, and no grade 4 IRRs were reported. IRRs resolved in all cases, except in 1 patient who discontinued treatment because of laryngeal edema during the first infusion. Two different infusion schedules investigated the feasibility of shortened first infusion times. IRR rates during the first infusion were higher with the accelerated schedule. With the exception of the patient with laryngeal edema, all patients recovered and resumed subsequent treatment cycles.
Blood transfusions are typically required for relapsed patients treated with lenalidomide and dexamethasone, especially among patients who experience anemia. Daratumumab can cause panreactivity in blood crossmatching assays as a result of the drug’s affinity to CD38 on red blood cells.24,25 A range of methods have been successfully deployed to overcome this interference, including phenotyping patients before the first daratumumab dose, incubating red blood cells with dithiothreitol, using antidaratumumab idiotype antibodies, and genotyping.26 No hemolysis was observed in any patient who received blood transfusions.
The synergy between daratumumab and lenalidomide/dexamethasone may be due, in part, to daratumumab’s role, not just in immune-mediated activities (complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis, and antibody-dependent cell-mediated cytotoxicity),4-6 but also in immunomodulation via T-cell expansion and activation and mitigation of immunosuppression.9 Lenalidomide increases T-cell and natural killer cell function through degradation of the transcriptional regulators ikaros and aiolos, which stimulate interleukin-2 production and thereby promote immune cell expansion and activation.27 In patients treated with daratumumab monotherapy, other observations included reduction in CD38+ immune-suppressive populations (subsets of regulatory T cells, regulatory B cells, and myeloid-derived suppressor cells), along with concomitant increases in CD4+ and CD8+ absolute T cells in the periphery and bone marrow.9 In addition, CD38 upregulation has been previously reported with IMiDs, including lenalidomide, on MM cell lines and may represent a class effect of IMiDs.28,29 This effect may further enhance the efficacy of daratumumab when used in combination with lenalidomide/dexamethasone.
Recently, a prespecified interim analysis of POLLUX, a randomized, open-label, phase 3 study of daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in relapsed or refractory myeloma patients with ≥1 prior line of therapy, revealed a highly significant improvement in PFS for the triplet combination versus the control, along with higher rates of deep responses and minimal residual disease negativity.30 The safety profiles were found to be consistent with those observed in this study, with no new safety signals being identified. Together, these results highlight the favorable benefit/risk profile of this daratumumab-based combination regimen for relapsed or relapsed and refractory MM. An ongoing phase 3 study will examine whether the benefit of this combination can be extended to newly diagnosed myeloma patients (ClinicalTrials.gov identifier: NCT02252172).
The investigators had full access to all data and analyses and were not restricted by confidentiality agreements.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the patients who participated in this study and their families, as well as the study co-investigators, research nurses, and coordinators at each of the clinical sites, and the Independent Review Committee.
This study was funded by Janssen Research & Development, LLC and Genmab A/S. Medical writing and editorial assistance were provided by Erica Chevalier-Larsen and Christopher Jones of MedErgy and were funded by Janssen Global Services, LLC.
The investigators had full access to all data and analyses and were not restricted by confidentiality agreements.
Authorship
Contribution: All authors developed the manuscript, provided final submission approval, and confirmed that the protocol was followed and that the data were accurate and complete.
Conflict-of-interest disclosure: T.P. received research funding from Roche, Novartis, Janssen, and Celgene; and was a member of advisory committees for Janssen, Celgene, and Genmab. P.G. received honoraria from Amgen. J.P.L. received research funding from Novartis, Onyx Pharmaceuticals, Celgene, and Millennium Pharmaceuticals. A.P. was a consultant for and received honoraria from Novartis, Sanofi, Celgene, Millennium Pharmaceuticals, Amgen, Bristol-Myers Squibb, Genmab, Janssen-Cilag, and Onyx Pharmaceuticals. S.L. and L.B. were employed by Genmab. J.W., A.K.S., M.E.G., C.d.B., N.Z.K., H.Y., P.L.C., and T.A. were employed by Janssen. H.M.L. received honoraria and research funding from Genmab and Janssen and honoraria from Amgen. P.G.R. was a member of advisory committees for Bristol-Myers Squibb, Celgene, Novartis, Millennium Takeda, and Johnson & Johnson. The remaining authors declare no competing financial interests.
Corresponding author: Torben Plesner, Department of Haematology, Vejle Hospital and University of Southern Denmark, Kabbeltoft 25, Vejle 7100, Denmark; e-mail: torben.plesner@rsyd.dk.