In this issue of Blood, Aguilar et al demonstrate that megakaryocytes adapt to the “stiffness” of their microenvironment and that re-creating the physical constraints encountered in native bone marrow dramatically improves in vitro megakaryocyte differentiation and proplatelet formation.1
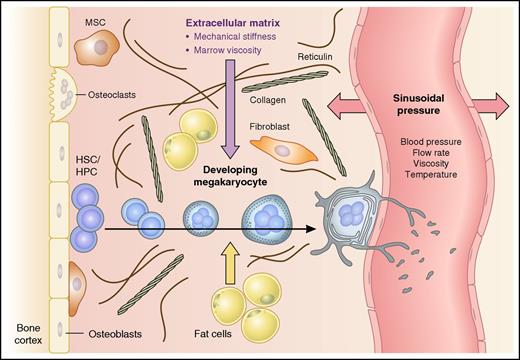
Elements that influence the structural rigidity of the bone marrow microenvironment. Model shows a developing megakaryocyte migrating from the endosteal bone lining to a bone marrow sinusoid. Cellular expansion, polyploidization, and development of the intracellular demarcation membrane system (DMS) are shown, with shedding of platelets from proplatelet extensions within the vessel lumen. Factors that may influence the mechanical forces (block arrows) within bone marrow include marrow fat content, extracellular matrix components (eg, collagen, fibronectin, and vitronectin), and the hydrostatic pressure of sinusoids, which in turn is determined by blood pressure, flow rate, blood viscosity, and temperature. HPC, hematopoietic progenitor cell; HSC, hematopoietic stem cell; MSC, mesenchymal stem cell.
Elements that influence the structural rigidity of the bone marrow microenvironment. Model shows a developing megakaryocyte migrating from the endosteal bone lining to a bone marrow sinusoid. Cellular expansion, polyploidization, and development of the intracellular demarcation membrane system (DMS) are shown, with shedding of platelets from proplatelet extensions within the vessel lumen. Factors that may influence the mechanical forces (block arrows) within bone marrow include marrow fat content, extracellular matrix components (eg, collagen, fibronectin, and vitronectin), and the hydrostatic pressure of sinusoids, which in turn is determined by blood pressure, flow rate, blood viscosity, and temperature. HPC, hematopoietic progenitor cell; HSC, hematopoietic stem cell; MSC, mesenchymal stem cell.
Megakaryocytes are one of the rarest cell types in bone marrow and yet collectively generate ∼1011 platelets each day.2 One platelet transfusion unit contains ∼3 to 4 × 1011 platelets, and significant advances have been made in the large-scale production of megakaryocytes and platelets from human pluripotent stem cells.3 Despite the incredible physiological capacity for platelet production, ex vivo platelet biogenesis from megakaryocytes differentiated in standard liquid culture systems from hematopoietic progenitors remains inefficient. One explanation for this is that key environmental components and/or mechanical forces encountered in native bone marrow are lacking in standard “2-dimensional” liquid culture systems.
In addition to secreted factors, metabolic components, and extracellular matrix factors, bone marrow provides a mechanical environment that is shaped by local hydrostatic pressure, sheer stress and viscosity (see figure).4 The structural scaffolds provided by specific bone marrow “niches”5 regulate the differentiation of all blood cell lineages and are especially important for megakaryopoiesis and thrombopoiesis. Developing megakaryocytes migrate from the bone lining to be close to specialized marrow sinusoids, where they reach “proplatelet” extensions through the vessel wall and release platelets into the circulation in response to hydrodynamic sheer.6,7 As megakaryocytes mature in vivo, they massively increase their cell size and nuclear content by endomitosis and polyploidization.2 A distinct network of cytoplasmic intracellular membranes termed the demarcation membrane system (DMS) develops, from which cytoplasmic territories and subsequently proplatelet extensions and platelet buds are eventually formed. Low-ploidy megakaryocytes (2N-4N) generate fewer platelets than higher-ploidy megakaryocytes (32N-64N). Megakaryocytes cultured in vitro fail to achieve the high ploidy levels observed in vivo and do not efficiently generate platelets, suggesting that the mechanical forces of the environment in which megakaryocytes differentiate are important for megakaryocyte maturation.
Previous attempts to design 3-dimensional (3D) in vitro systems mimicking the native environment in which megakaryocytes mature and produce platelets have incorporated artificial scaffolds and sheer forces in addition to cytokines, matrix components and/or endothelial cells.8,9 For example, Balduini’s group developed a 3D bioreactor using silk-based vascular tubes.9 Italiano et al designed a microfluidic bioreactor incorporating sheer forces, matrix interactions, and simulating bone marrow stiffness that also enabled high-resolution live-cell microscopy.8 In the current study, Aguilar et al compared murine megakaryocytes differentiated in vitro in liquid medium and in methylcellulose (MC) hydrogels with megakaryocytes harvested directly from mouse bone marrow.1 They tested hydrogels consisting of 2% and 2.5% MC, as these concentrations had a mechanical stiffness similar to that estimated for bone marrow (15-300 Pa), although the stiffness of 2.5% MC was 10-fold higher than 2% MC (300-600 Pa vs 30-60 Pa, respectively).
Electron microscopy showed that while liquid-cultured megakaryocytes had an abnormally developed DMS with poor demarcation of cytoplasmic territories, those grown in 2% MC developed a DMS that closely resembled that seen in megakaryocytes isolated from bone marrow. Fourfold fewer megakaryocytes grew in 2.5% MC gels than in 2% MC gels, and 2.5% MC gel–derived megakaryocytes were smaller with a poorly developed DMS, indicating that megakaryocytes are highly sensitive to the specific viscoelastic properties of their microenvironment. Cells cultured in 2% MC also achieved higher ploidy, and importantly, the percentage of cells extending proplatelets was almost doubled in 2% MC as compared with liquid cultures.
Remarkably, the DMS rapidly disordered and cytoplasmic territories were lost within 2 hours when megakaryocytes were removed from MC hydrogels and resuspended in liquid culture. Two interesting hypotheses are raised by these observations. Firstly, differences in the mechanical stiffness between regions of bone marrow (eg, perivascular vs endosteal niches) may create areas that are more or less conducive to platelet production in vivo. Secondly, the rapid changes observed when megakaryocytes were switched from liquid culture to 2% MC hydrogels may occur physiologically, with dynamic remodelling of the DMS occurring as megakaryocytes experience fluctuations in extracellular tension and mechanical forces.
What is the relevance of these findings for patients with platelet disorders? A group of inherited thrombocytopenias are caused by mutations in the gene encoding myosin heavy chain 9 (MYH9), a cytoskeletal contractile protein, leading to defects in platelet production as well as characteristic inclusion bodies in leukocytes and renal and hearing abnormalities. Paradoxically, while Myh9−/− megakaryocytes show impaired proplatelet formation within bone marrow, in vitro cultured MYH9-deficient megakaryocytes show an increased capacity for proplatelet formation,10 suggesting that the defect caused by myosin deficiency is masked by in vitro liquid culture conditions. Aguilar et al found that myosin expression was absent from the cytoplasm in liquid-cultured megakaryocytes, and Myh9−/− murine megakaryocytes did not increase proplatelet formation in response to 2% MC gels as did wild-type megakaryocytes,1 indicating that myosin is required for megakaryocytes to adapt to the stiffness of their microenvironment. Further, nuclear accumulation of the transcription factor MKL1 occurred in response to culture in 2% MC, and treatment with an MKL1 inhibitor completely eliminated the increased proplatelet formation observed in the MC gels,1 suggesting a role for the MKL1 pathway.
Bone marrow is a unique and dynamic mechanical environment: a viscous organ confined by solid bone. Mechanical forces exerted on differentiating hematopoietic cells are likely to be dynamic, changing in response to blood flow rates and volume and therefore factors such as physical activity and temperature (see figure). This study emphasizes the importance of considering the viscoelastic properties of any in vitro culture system. This may be especially relevant for the study of megakaryocyte and platelet disorders due to abnormal cytoskeletal proteins. Further, it highlights interesting questions regarding how physiological fluctuations, as well as pathologies such as myelofibrosis, alter physical forces within bone marrow and thereby may influence hematopoiesis.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal