Abstract
A normal hemostatic response to vascular injury requires both factor VIII (FVIII) and von Willebrand factor (VWF). In plasma, VWF and FVIII normally circulate as a noncovalent complex, and each has a critical function in the maintenance of hemostasis. Furthermore, the interaction between VWF and FVIII plays a crucial role in FVIII function, immunogenicity, and clearance, with VWF essentially serving as a chaperone for FVIII. Several novel recombinant FVIII (rFVIII) therapies for hemophilia A have been in clinical development, which aim to increase the half-life of FVIII (∼12 hours) and reduce dosing frequency by utilizing bioengineering techniques including PEGylation, Fc fusion, and single-chain design. However, these approaches have achieved only moderate increases in half-life of 1.5- to 2-fold compared with marketed FVIII products. Clearance of PEGylated rFVIII, rFVIIIFc, and rVIII-SingleChain is still regulated to a large extent by interaction with VWF. Therefore, the half-life of VWF (∼15 hours) appears to be the limiting factor that has confounded attempts to extend the half-life of rFVIII. A greater understanding of the interaction between FVIII and VWF is required to drive novel bioengineering strategies for products that either prolong the survival of VWF or limit VWF-mediated clearance of FVIII.
Introduction
Factor VIII (FVIII) is a glycoprotein synthesized by sinusoidal and vascular endothelial cells in the liver and lung1,2 that is critical for hemostasis.3 Normal hemostatic responses to vascular injury require both FVIII and its physiologic partner, von Willebrand factor (VWF).4 In plasma, VWF and FVIII circulate as a noncovalent complex that regulates platelet aggregation and clot formation. Each protein is a separate gene product but the processes they regulate are coordinated and critical to maintenance of hemostasis. VWF is required for platelet adhesion to subendothelium, and for normal FVIII survival in circulation.4 Deficiencies or structural defects in FVIII or VWF are responsible for hemophilia A (HA) and von Willebrand disease (VWD), respectively.5,6 Herein, we review the FVIII-VWF interaction and its implications for the treatment of HA, including FVIII half-life, clearance, and immunogenicity. A better understanding of this interaction is critical to facilitate development of improved therapies.
Biology of VWF and FVIII
Although it is now well recognized that VWF and FVIII are 2 separate gene products, the presence of a VWF-FVIII protein complex led to early misunderstandings about their relationship. Hemophilia was recognized in the Talmud >1700 years ago7 and was first effectively treated using whole-blood transfusion in the 1840s.8 VWD was first described by Erik von Willebrand and the disorder was termed pseudohaemophilia.9 In the 1960s, antihemophilic factor, which is synonymous with FVIII, was recognized as the missing clotting factor in both HA and severe VWD; it was also recognized that another vascular component was missing in VWD that was present in patients with HA.10 In the 1970s, sometimes heated debates ensued as to whether the clotting factor (FVIII) and the vascular factor (VWF) were on 111 or 212 molecules.
Once VWF was recognized as a distinct protein, a flurry of studies demonstrated that it was synthesized in 2 cell types: megakaryocytes and endothelial cells, processed and then stored in platelet α-granules and endothelial Weibel-Palade bodies, respectively.13-15 The intracellular synthesis and trafficking of VWF is complex and has been best characterized in endothelial cells. Following synthesis of pro-VWF (propeptide and VWF monomer), C-terminal dimerization takes place cotranslationally in the endoplasmic reticulum before transport to the Golgi where the dimers undergo further carbohydrate modification, multimerization, VWF-propeptide cleavage, and storage. The endothelium stores high-molecular-weight VWF multimers16 that can be released by 1-deamino-8-D-arginine vasopressin (DDAVP; desmopressin), which binds to the vasopressin V2 receptor and induces release of VWF multimers into plasma.17 DDAVP releases VWF from endothelial cells but not from platelets.
Synthesis and storage of VWF in megakaryocytes/platelets is more complex. Although α-granules contain VWF, other stored proteins are much more heterogeneous.18,19 Platelet α-granule contents are released during platelet aggregation in response to agonists such as arachidonic acid, adenosine 5′-diphosphate, collagen, and epinephrine. VWF is critical for both recruitment and activation of platelets. At sites of vascular injury, plasma VWF binds to exposed collagen via 2 collagen-binding sites. The A1 domain binds to collagen IV and VI, and the A3 domain binds to collagen I and III.20 The immobilized VWF then undergoes shear-induced rearrangement21 to spontaneously recruit platelets by adhesion through the glycoprotein Ib-IX receptor22 expressed on platelets. The recruited platelets then undergo activation through fibrinogen- and VWF-dependent interactions with αIIbβ3, which induces platelet aggregation.23,24
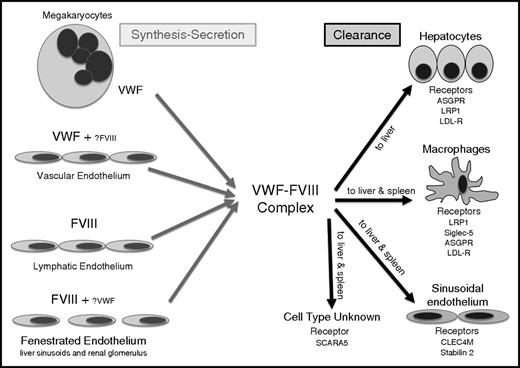
The biosynthesis of FVIII is complex, and some questions about its sites of synthesis remain unanswered (Figure 1). Early transplantation studies in dogs (and subsequently in humans) demonstrated that transplantation of a normal liver to a HA patient can normalize plasma FVIII levels.25,26 Although these studies suggested that liver was a major site of FVIII synthesis, other transplantation studies suggested that FVIII was synthesized by spleen and lymphatic tissue.27,28 Although liver transplantation has resulted in normalized plasma FVIII levels and a lifelong cure for many patients, studies of transplant recipients suggest that the DDAVP-releasable pool of FVIII is not restored by liver transplant.29 Furthermore, significant extrahepatic FVIII synthesis was demonstrated in a normal subject who received a liver from a HA patient and who did not then experience reduced plasma FVIII levels.30 It has also been demonstrated that FVIII is synthesized by sinusoidal and microvascular endothelial cells,1,2 and in situ studies identified FVIII messenger RNA in endothelial cells.31 Finally, 2 recent studies using murine conditional knockout approaches indicated endothelial cells are the major, if not exclusive, source of FVIII,32,33 and gene transfer of FVIII to endothelial cells in mice normalized plasma FVIII and restored the releasable FVIII pool.34 Recent studies have also documented the presence of FVIII on the VWF strings that are released from the endothelium.35 However, the documentation of FVIII expression in lymphatic endothelium and certain types of fenestrated endothelium (in the liver sinusoids and renal glomerulus), with little or no coexpression of VWF,36 suggests that there is still more to learn about the precise endothelial sources of production of these 2 proteins. Nevertheless, it seems clear that different endothelial beds show differential expression of VWF and FVIII.
Details of the sites of synthesis and clearance of VWF and FVIII. Although the synthesis of VWF has long been known to be the vascular endothelium and megakaryocytes, the location of FVIII expression has only recently been confirmed in some types of endothelial cell: in fenestrated forms of endothelium (liver sinusoidal endothelium and glomerular endothelium), in lymphatic endothelium, and in some high endothelial venules. In most forms of endothelium, VWF and FVIII are not coexpressed. Clearance of VWF and FVIII occurs most frequently as a complex, in the sinusoids of the liver and spleen where a range of lectin and scavenger receptors expressed on macrophages, sinusoidal endothelium, and hepatocytes bind to and internalize the 2 proteins. Protein clearance is influenced by factors such as shear, desialylation, and protein sequence variants. SCARA5, scavenger receptor class A, member 5.
Details of the sites of synthesis and clearance of VWF and FVIII. Although the synthesis of VWF has long been known to be the vascular endothelium and megakaryocytes, the location of FVIII expression has only recently been confirmed in some types of endothelial cell: in fenestrated forms of endothelium (liver sinusoidal endothelium and glomerular endothelium), in lymphatic endothelium, and in some high endothelial venules. In most forms of endothelium, VWF and FVIII are not coexpressed. Clearance of VWF and FVIII occurs most frequently as a complex, in the sinusoids of the liver and spleen where a range of lectin and scavenger receptors expressed on macrophages, sinusoidal endothelium, and hepatocytes bind to and internalize the 2 proteins. Protein clearance is influenced by factors such as shear, desialylation, and protein sequence variants. SCARA5, scavenger receptor class A, member 5.
It is reasonable to ask where these 2 proteins first meet to form the noncovalent complex that circulates in plasma (Figure 1). In humans, the DDAVP-releasable FVIII pool is dependent on the in vivo synthesis of both VWF and FVIII.37 If FVIII expression is induced in a cell that synthesizes and stores VWF, FVIII will follow VWF and be stored and released as a noncovalent complex similar to that found in normal plasma.38 This is probably what happens in some but not necessarily all endothelial cells. Type 2N VWD is associated with mutations in its D′D3 domain that interfere with FVIII binding.39,40 When not bound to VWF, FVIII is cleared rapidly, as in type 3 VWD. However, DDAVP induces release of both VWF and FVIII, but following release the FVIII half-life is 2 hours whereas VWF has a normal half-life of 12 hours in type 2N VWD.41 This suggests that intracellularly, in the acidic environment of the late Golgi, there is normal association and storage of the VWF-FVIII noncovalent complex that is disrupted at the neutral pH of plasma following release.42
Clinical implications for hemophilia A
The fact that FVIII and VWF are colocalized in storage granules within endothelial cells has implications for the selection of target tissues for FVIII gene therapy studies. Despite the investigation of various strategies to deliver the complementary DNAs (cDNAs) for either FVIII or FIX in human gene therapy trials, success has only been achieved using a liver-targeted recombinant adeno-associated virus vector to deliver the FIX cDNA to individuals with hemophilia B.43 Initial attempts at gene therapy for HA included in vivo delivery of FVIII using retrovirus or “gutless” adenovirus vectors and omental implantation of ex vivo transduced autologous fibroblasts. However, these strategies have not led to sustained FVIII expression or therapeutic FVIII plasma levels. These attempts have been hampered by the large size of the FVIII cDNA and relatively inefficient expression in target tissues. To overcome these challenges, current strategies involve bioengineering the FVIII cDNA to remove the B domain, adding targeted consensus sequences for asparagine-linked glycosylation, and optimizing the transgene codons.44 Investigators have also been exploring alternative target tissues, in an effort to mimic a more physiologic source for FVIII expression in vivo. In 1 study, human umbilical vein endothelial cells were transduced with a retroviral FVIII (B-domain deleted) construct.45 The investigators demonstrated that FVIII colocalized with VWF in Weibel-Palade bodies, and the human umbilical vein endothelial cells, upon agonist-induced stimulation, displayed a parallel release of FVIII and VWF, suggesting that this approach reconstituted the normal physiologic storage pool of FVIII-VWF complexes. Blood outgrowth endothelial cells have also been transduced ex vivo with viral vectors, and the transduced blood outgrowth endothelial cells can be delivered systemically, resulting in their transplantation into several organs.46 This strategy also achieved FVIII/VWF colocalization. In addition, it has been demonstrated that targeting FVIII expression to platelets results in FVIII storage together with VWF in platelet α-granules and corrects the murine hemophilia A phenotype even in the presence of high-titer anti-FVIII inhibitory antibodies (inhibitors).47
FVIII and VWF structural biology
FVIII spends virtually its entire life cycle interacting with other proteins and membrane surfaces. Recent studies have expanded our understanding of the structures and interactions of FVIII and VWF, yielding clinical insights. For example, mutagenesis studies and patient-derived data are defining the roles of specific amino acids in the activities of both proteins. The structures and affinities of FVIII binding to its various partners, though informative, must be interpreted with caution, as interactions between isolated components of, for example, the intrinsic tenase complex may differ somewhat from those that occur physiologically, at wound sites and under shear forces (Figure 2). Therefore, the growing body of FVIII structural studies (crystallographic,48-50 fluorescence resonance energy transfer,51 cryoelectron microscopic,52 etc) are best considered a series of “snapshots” from the ensemble of conformations accessible to this multidomain protein.
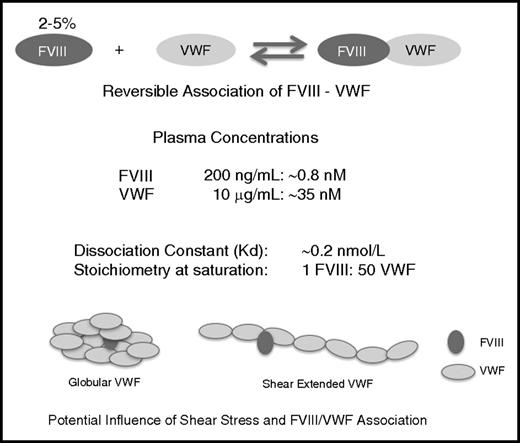
Dynamic equilibrium between VWF and FVIII and details of the VWF-FVIII association under normal conditions of synthesis, secretion, and clearance. Whereas the vast majority of VWF circulates as an FVIII-free protein in the circulation, the opposite is true for FVIII with 95% to 98% being in complex with VWF.128 Although of relatively high affinity (KD, 0.2 nM), complex formation is characterized by a temperature-sensitive highly dynamic equilibrium, with rapid association and dissociation rate constants (2-4 × 106 M−1 s−1 and 0.3-6 × 10−3 s−1), respectively.129 The influence of shear on the VWF-FVIII association and configuration is unresolved but may play a role in modulating clearance and immunogenicity.
Dynamic equilibrium between VWF and FVIII and details of the VWF-FVIII association under normal conditions of synthesis, secretion, and clearance. Whereas the vast majority of VWF circulates as an FVIII-free protein in the circulation, the opposite is true for FVIII with 95% to 98% being in complex with VWF.128 Although of relatively high affinity (KD, 0.2 nM), complex formation is characterized by a temperature-sensitive highly dynamic equilibrium, with rapid association and dissociation rate constants (2-4 × 106 M−1 s−1 and 0.3-6 × 10−3 s−1), respectively.129 The influence of shear on the VWF-FVIII association and configuration is unresolved but may play a role in modulating clearance and immunogenicity.
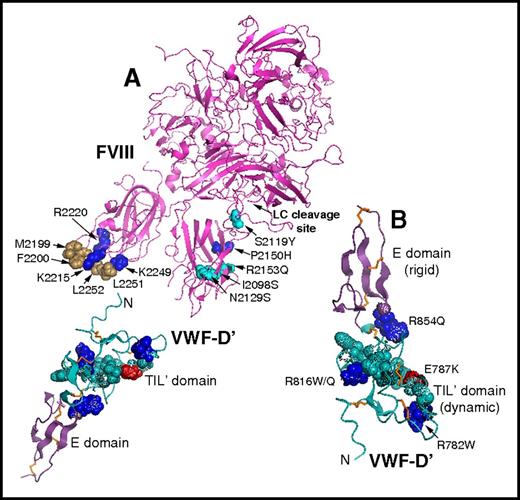
Binding of FVIII to VWF is mediated by noncovalent interactions between the FVIII-C1 and FVIII-C2 domains, an acidic peptide (FVIII-ap) at its light-chain N terminus, and the VWF D′D3 region (VWF-D′D3).53 VWF-D′D3 comprises the N-terminal region of monomeric VWF created during proteolysis and assembly to create multimeric VWF. Studies investigating the crystal structure of human FVIII-C2,49 homology-modeled FVIII-C1,54 and mutations in both domains have suggested that certain amino acid substitutions affect residues that directly mediate FVIII-VWF interaction, whereas others result in protein misfolding.54 Medium-resolution crystal structures show no electron density for FVIII-ap, indicating that it is flexible.48,50 A VWF-D′ solution structure55 (Figure 3), as well as modeling based on single-particle electron microscopy of complexes formed by FVIII and VWF-D′D3 domains,56,57 have allowed further analysis of the FVIII-VWF interface and of VWF-D′ mutations associated with reduced FVIII-VWF affinity and type 2N VWD. These studies have shown that the positively charged VWF-D′ surface is probably a binding site for FVIII-ap.56,57 More detailed models are on the horizon, as the 3-dimensional structures suggest additional mutagenesis studies to identify specific residues that mediate FVIII-VWF binding.
VWF-D′ solution structure. FVIII and VWF-D′ domain structures. Residues where substitutions affected or would be expected to affect binding affinity are shown in spherical representation (blue, positively charged; red, negatively charged; brown, hydrophobic; turquoise, neutral). (A) Hydrophobic FVIII-C2 residues M2199, F2200, L2251, and L2252 interact with VWF, whereas flanking surface-exposed residues R2215, R2220, and K2249 make this FVIII-C2 region positively charged. Five noncysteine hemophilic FVIII-C1 domain amino acid substitutions that affected FVIII-VWF binding (http://www.factorviii-db.org/) are indicated. The cleavage site for the FVIII light-chain (LC) acidic peptide is also indicated. VWF-D′ is oriented with its flexible TIL′ domain approaching FVIII. (B) VWF-D′ is oriented here with its rigid E domain at the top. Noncysteine amino acids whose substitutions are associated with type 2N VWD are shown in spherical representation, and those affecting charged side chains are labeled. Disulfide bonds are shown in orange stick representation.
VWF-D′ solution structure. FVIII and VWF-D′ domain structures. Residues where substitutions affected or would be expected to affect binding affinity are shown in spherical representation (blue, positively charged; red, negatively charged; brown, hydrophobic; turquoise, neutral). (A) Hydrophobic FVIII-C2 residues M2199, F2200, L2251, and L2252 interact with VWF, whereas flanking surface-exposed residues R2215, R2220, and K2249 make this FVIII-C2 region positively charged. Five noncysteine hemophilic FVIII-C1 domain amino acid substitutions that affected FVIII-VWF binding (http://www.factorviii-db.org/) are indicated. The cleavage site for the FVIII light-chain (LC) acidic peptide is also indicated. VWF-D′ is oriented with its flexible TIL′ domain approaching FVIII. (B) VWF-D′ is oriented here with its rigid E domain at the top. Noncysteine amino acids whose substitutions are associated with type 2N VWD are shown in spherical representation, and those affecting charged side chains are labeled. Disulfide bonds are shown in orange stick representation.
FVIII is sulfated at 6 tyrosine residues, and these are key modulators of its extracellular protein-protein interactions. Sulfation of FVIII-Tyr1680 is required for efficient VWF binding, increasing the FVIII-VWF affinity fivefold.58 Full sulfation at Tyr1680 appears to be dependent on the cell line and expression conditions. Nielsen and colleagues examined extracted ion chromatograms showing sulfated and nonsulfated (minor or absent peak) Tyr1680-containing peptides.59 Plasma-derived FVIII and recombinant FVIII (rFVIII) from human HEK293 cells demonstrated only a sulfated peak,60 whereas rFVIII expressed by rodent cells exhibited both a sulfated peak and nonsulfated peaks. Likewise, a B-domain–deleted rFVIII expressed in Chinese hamster ovary (CHO) cells showed full Tyr1680 sulfation, suggesting that culture conditions may influence the efficiency of this modification. These studies highlight that choices made in cell production and design of FVIII constructs can have important implications with regard to optimizing the FVIII-VWF interaction.
FVIII immunogenicity: role for VWF
Development of an immune response against FVIII complicates the treatment of ∼30% of patients with HA.61 Neutralizing antibodies (“inhibitors”) are a more significant problem in HA than in VWD because approximately half of severe HA cases are caused by mutations that result in no circulating FVIII protein, whereas type 3 VWD (complete lack of VWF) is extremely rare. Patients with a circulating dysfunctional FVIII or VWF (eg, with a missense substitution) are far more likely to have functional immune tolerance to therapeutic FVIII. Patients who develop inhibitors typically have antibodies that bind to the FVIII-A2 and FVIII-C2 domains,62 and there has been substantial progress in characterizing FVIII B-cell epitopes.63-67
Risk factors for development of inhibitors in HA include genetic factors such as the type of F8 gene mutation, the severity of hemophilia, HLA haplotype, and polymorphisms in genes involved in immunoregulation68 as well as environmental factors such as the intensity of FVIII exposure and concomitant immunologic “danger signals.”69 Identification of risk factors related to rFVIII vs plasma-derived FVIII has been more controversial. Product-specific differences that have been examined include amino acid sequence alterations, including B-domain deletion/truncation, glycosylation patterns, and a potential protective role of VWF.70,71
Glycosylation patterns for rFVIII depend on the cell line from which the protein is produced, and they tend to differ from those of plasma-derived products. These differences may contribute to immunogenicity, although other factors cannot be ruled out, including an immunomodulatory role for VWF.72 A rFVIII has been produced in a human cell line with the aim of retaining human-specific posttranslational modifications, including its glycosylation profile.60 To date, clinical studies with this human cell line-derived rFVIII, involving 59 and 32 previously treated children and adult hemophiliacs, respectively, have shown no FVIII inhibitors.73,74
As the chaperone of FVIII in plasma, the role of VWF in FVIII immunogenicity cannot be ignored. It has been proposed that VWF may serve an immunoprotective role in 2 ways. First, VWF reduces FVIII endocytosis by antigen-presenting cells and a recent study has shown that VWF also modulates FVIII peptide presentation by dendritic cells.75-77 Second, by increasing the FVIII half-life in circulation, VWF may increase contact time and processing of FVIII by B cells in the splenic marginal zone, promoting immunoregulatory mechanisms, and resulting in tolerance. Immunodominant B-cell epitopes have been described in the FVIII A2 and C2 domains.78 Binding of FVIII to VWF is postulated to shield 1 or more C2 domain epitopes and decrease uptake of FVIII by antigen-presenting cells. However, a study by Meeks and colleagues found that neutralizing anti-C2 antibodies still formed when FVIII was unable to dissociate from VWF.79 The same study also found evidence that in the absence of VWF, FVIII may be cleared by alternative receptors through which it is degraded in a process that does not include antigen presentation. Thus, it appears that FVIII immunogenicity may be both positively and negatively affected by its association with VWF. FVIII protein content in FVIII products may also affect immunogenicity. One study showed that rFVIII preparations contained significantly more FVIII protein per international unit (IU) than plasma-derived FVIII concentrates, and that only ∼80% of the rFVIII could bind to VWF.80 This unbound rFVIII may have been denatured or damaged, which could cause increased immunogenicity.
Preclinical and clinical data further support the notion that the presence of VWF in FVIII preparations is associated with reduced FVIII immunogenicity.72,81,82 In a systematic review of previously untreated patients (PUPs) with HA, the risk of inhibitor development was greater with rFVIII than with VWF-containing plasma-derived FVIII (27.4% [95% confidence interval (CI), 23.6-31.5] vs 14.3% [95% CI, 10.4-19.4], respectively).83 In addition, using a combination of retrospective and prospective data, the relative risk for inhibitor development in PUPs treated with rFVIII was higher than for PUPs who received plasma-derived FVIII (relative risk = 2.4; 95% CI, 1.0-5.8; P = .049).84 A note of caution in the interpretation of PUP studies is that, in many instances, some of the PUPs have received a small number of blood-product infusions prior to study inclusion (minimally treated patients). This factor may confound interpretation of the origin of the FVIII immunogenic stimulus.
It should be noted that other studies have reported conflicting results. A large retrospective analysis of data on 316 PUPs enrolled in the Concerted Action on Neutralizing Antibodies in severe haemophilia A (CANAL) cohort study found that plasma-derived FVIII with considerable quantities of VWF carried the same inhibitor risk as rFVIII products.85 A meta-analysis of 28 prospective studies investigating inhibitor development in 1421 PUPs with severe HA reached similar conclusions, finding no statistically significant difference in inhibitor development between plasma-derived FVIII and rFVIII or between different classes of rFVIII.71 Most recently, the Study on Inhibitors in Plasma-Product Exposed Toddlers (SIPPET) trial, the first prospective randomized study to address this issue, has reported a 1.87-fold increase in inhibitors in PUPs treated with rFVIII compared with plasma-derived FVIII.86 The study includes 251 previously untreated HA boys (<6 years of age) randomly assigned to receive a VWF-containing plasma-derived FVIII product or a rFVIII product and followed for 50 exposure days for inhibitor development. This study presents the first clear evidence of a differential immunogenicity risk associated with rFVIII products. Although several issues have been raised by this study, including whether the results from a geographically unusual study population (73% of subjects derive from India, Egypt, and Iran) can be extrapolated more widely, the limit of follow-up to 50 exposure days, and the choice of a lower-than-usual inhibitor titer threshold (0.4 Bethesda units), the study results have nevertheless caused a major debate about the optimal FVIII product to begin therapy in PUPs. Importantly, the mechanistic basis of a differential immunogenic tendency for rFVIII products remains without explanation.
Inhibitor development requires activation of CD4+ T-effector cells, and several immunodominant T-cell epitopes have been identified in FVIII.87-89 Characterization of FVIII-specific T-cell clones and lines, and their use in studies exploring cell-based immunotherapies to promote immune tolerance to FVIII, are suggesting novel approaches to reduce the incidence of inhibitors.90,91 Animal models remain essential for mechanistic studies,92 and preclinical examination has demonstrated potential immune-regulatory properties of Fc fusion proteins93 and the possible mitigation of FVIII immunogenicity with the long-term persistence of circulating FVIII levels achieved by gene transfer either alone94 or through the generation of T-regulatory cells.95
Clearance of the FVIII-VWF complex
Because FVIII-VWF circulates as a protein complex, assessment of how the individual constituents are removed from circulation is complex. Human and animal studies have demonstrated that FVIII half-life is reduced about sixfold in the absence of VWF, whereas the VWF half-life is unaffected by the presence of FVIII. This is compatible with the view that most FVIII molecules are eliminated while complexed to VWF, and further suggests that VWF protects FVIII from premature clearance. To understand FVIII clearance it is thus essential to understand VWF catabolism.
Interestingly, VWF clearance varies significantly between individuals. This is not only true when assessing endogenous VWF survival following desmopressin treatment (range, 4-26 hours), but also after infusing exogenous VWF concentrates (range, 4-157 hours).96 This variability likely originates from both VWF and non-VWF-related factors. Three categories of potential parameters that could modulate VWF clearance are summarized in Table 1: patient-related, receptor-related, and VWF-related. To illustrate how these parameters influence VWF catabolism, the interaction between VWF and some of its potential clearance receptors (Table 2) will be discussed.
Parameters that potentially modulate clearance of VWF
| Parameters . | Affects VWF interaction with . |
|---|---|
| Patient-related parameters | |
| • Body weight | • ASGPR |
| • Age | • ASGPR |
| • Diet | • ASGPR |
| • ABO antigen status | • ? |
| • Vascular integrity | • LRP1 |
| • Inflammatory state | • ASGPR |
| Receptor-related parameters | |
| • Repertoire, expression level, activity | • ? |
| • Sequence variations | • CLEC4M, LRP1 |
| VWF-related parameters | |
| • Sequence variations (polymorphisms, mutations) | • LRP1 |
| • Glycosylation variations | • ASGPR, Siglec-5 |
| • Proteins associated with VWF (FVIII, osteoprotegerin, galectins, etc) | • ? |
| • Activation state, unfolding | • LRP1 |
| Parameters . | Affects VWF interaction with . |
|---|---|
| Patient-related parameters | |
| • Body weight | • ASGPR |
| • Age | • ASGPR |
| • Diet | • ASGPR |
| • ABO antigen status | • ? |
| • Vascular integrity | • LRP1 |
| • Inflammatory state | • ASGPR |
| Receptor-related parameters | |
| • Repertoire, expression level, activity | • ? |
| • Sequence variations | • CLEC4M, LRP1 |
| VWF-related parameters | |
| • Sequence variations (polymorphisms, mutations) | • LRP1 |
| • Glycosylation variations | • ASGPR, Siglec-5 |
| • Proteins associated with VWF (FVIII, osteoprotegerin, galectins, etc) | • ? |
| • Activation state, unfolding | • LRP1 |
Potential clearance receptors for FVIII and VWF
| Receptor* . | FVIII† . | Clearance?‡ . | VWF† . | Clearance?‡ . |
|---|---|---|---|---|
| LRP1 | Yes | Yes | Yes | Yes (requires VWF unfolding) |
| LDL-R | Yes | Yes | Unknown | Unknown |
| ASGPR | Yes | Yes (hyposialylated FVIII) | Yes | Yes (hyposialylated VWF) |
| CD206 | Yes | Probably not (antigen presentation) | No | No |
| Siglec-5 | Yes | Yes | Yes | Yes |
| CLEC4M | Unknown | Unknown | Yes | Yes |
| STAB2 | Yes | Yes | Yes | Yes |
| SCARA5 | Unknown | Unknown | Yes | Unknown |
| HSPGs | Yes | Possibly | Yes | Unlikely |
| Receptor* . | FVIII† . | Clearance?‡ . | VWF† . | Clearance?‡ . |
|---|---|---|---|---|
| LRP1 | Yes | Yes | Yes | Yes (requires VWF unfolding) |
| LDL-R | Yes | Yes | Unknown | Unknown |
| ASGPR | Yes | Yes (hyposialylated FVIII) | Yes | Yes (hyposialylated VWF) |
| CD206 | Yes | Probably not (antigen presentation) | No | No |
| Siglec-5 | Yes | Yes | Yes | Yes |
| CLEC4M | Unknown | Unknown | Yes | Yes |
| STAB2 | Yes | Yes | Yes | Yes |
| SCARA5 | Unknown | Unknown | Yes | Unknown |
| HSPGs | Yes | Possibly | Yes | Unlikely |
HSPG, heparin sulfate proteoglycan; SCARA5, scavenger receptor class A, member 5; STAB2, stabilin 2.
The 9 receptors listed have been demonstrated at some level to bind to and, in some cases, mediate clearance of FVIII and/or VWF.
Responses indicate evidence of receptor binding to the protein.
Responses indicate evidence for FVIII and VWF clearance by each receptor.
ASGPR
Like many proteins, VWF glycan structures are well sialylated,97,98 suggesting that the contribution of asialoglycoprotein receptor (ASGPR) to basal VWF clearance is limited. However, age, diet, and body weight can all reduce the extent of sialylation,99,100 consequently favoring ASGPR-mediated clearance. Furthermore, several infectious pathogens are known to release sialidases (or neuramidases in the case of influenza infections). These sialidases could remove sialic acids from VWF, thereby promoting binding to ASGPR. Indeed, infection with Streptococcus pneumoniae reduces VWF sialylation and is associated with increased VWF clearance.101 Thus, the patient’s inflammatory state may potentially affect VWF clearance. Finally, the extent of sialylation highly depends on the activity of the enzymatic glycosylation machinery. For instance, reduced activity of the sialyltransferase ST3Gal-IV results in decreased VWF sialylation and subsequently increased clearance.102 Conversely, increased sialylation activity may promote interactions with sialic acid–recognizing receptors, such as sialic acid binding immunoglobulin-like lectin 5 (Siglec-5).103
CLEC4M
Genome-wide association studies have shown that the CLEC4M gene is associated with VWF plasma levels,104 and later studies confirmed that the CLEC4M lectin receptor can bind to VWF and modulate VWF plasma levels.105 Interestingly, a relationship between the number of tandem repeats in the CLEC4M gene and levels of VWF activity and antigen was observed when analyzing plasma samples from VWD patients. Apparently, sequence variations in VWF receptors may modulate the efficiency by which they remove VWF from circulation.
LRP1
Although initially recognized as a FVIII clearance receptor,106,107 LRP1 has recently also been identified as a clearance receptor for VWF.108 Interestingly, VWF clearance by LRP1 depends strictly on the presence of increased hydrodynamic forces, suggesting that VWF needs to be unfolded in order to interact with LRP1. It has been hypothesized that changes in vascular integrity alter the shear stress to which VWF is exposed, thereby disturbing the clearance equilibrium between VWF and LRP1.108 Notably, although the contribution of LRP1 to VWF catabolism is modest (as for FVIII), it seems physiologically relevant, given that polymorphisms in the LRP1 gene are associated with VWF (and FVIII) plasma levels.109
Parameters that modulate VWF clearance undoubtedly also affect FVIII clearance, either indirectly or directly. Indeed, there is a large overlap between potential clearance receptors that recognize both FVIII and VWF (Table 2). Interestingly, VWF often interferes with FVIII binding to its proper receptor in vitro, as has been shown for ASGPR,110 Siglec-5,103 and LRP1.108 This does not mean that FVIII never interacts alone with these receptors. It is important to realize that the FVIII-VWF interaction is highly dynamic, with fast association and dissociation rates (Figure 2). This dynamic equilibrium implies that FVIII is frequently dissociated from VWF and therefore can bind to its proper clearance receptor. Moreover, it allows FVIII to move from 1 VWF molecule to another, a phenomenon that Yee and coworkers have elegantly shown occurs physiologically.111
Extended half-life of FVIII and VWF interaction
Understanding VWF clearance is of relevance with regard to the development of long-acting FVIII variants (Table 3). These variants display limited prolongation of their half-life, most likely because they still interact with endogenous VWF and are cleared as part of the FVIII-VWF complex. Efforts to prolong FVIII half-life by modifying VWF to modulate its clearance have also met with limited success because FVIII can distribute to unmodified, endogenous VWF. Therefore, longer-acting FVIII variants may require modifications that exclude association with endogenous VWF.
Novel, structurally altered rFVIII concentrates in development or recently approved, and the advantages and disadvantages of the technology used in their development
| Characteristic . | PEGylated FVIII . | Fc fusion platform . | Novel FVIII design . | ||
|---|---|---|---|---|---|
| N8-GP . | BAX 855* . | BAY 94-9027 . | rFVIIIFc† . | rVIII-SingleChain . | |
| Product description | B-domain–modified 40K O-glycoPEGylated rFVIII concentrate | 20-kDa PEGylated full-length rFVIII | BDD rFVIII with a site-specific branched 60-kDa PEG side chain | Recombinant BDD FVIII-Fc fusion protein | Recombinant single-chain FVIII construct |
| Spec.Act. 11 200 IU/mg‡ | Spec.Act. 8000 IU/mg‡ | Spec.Act. 9717 IU/mg‡ | Spec.Act. 9348 IU/mg‡ | Spec.Act. 12 000 IU/mg‡ | |
| Advantages of technology | • Well-established technology | • Established technology | • Increases the intrinsic stability of the FVIII molecule by reducing the potential dissociation of the heavy and light chains | ||
| • Improves solubility of proteins | • Extends half-life of proteins | • Increased affinity for and binding to VWF | |||
| • Extends half-life of non-PEG protein/drug | • May mitigate immunogenicity of the protein | ||||
| • Reduced kidney clearance because of larger hydrodynamic size | |||||
| • Protects the drug from proteolysis | |||||
| • May protect the drug from the immune system | |||||
| • Reduces aggregation | |||||
| Disadvantages of technology | • Random PEGylation (BAX 855) may result in loss or change in protein activity | • Potential for activation of the immune system (antibody-dependent or complement-dependent) | • Not specifically designed for extension of half-life | ||
| • Preexisting anti-PEG antibodies identified in the healthy population, which may result in the rapid clearance of PEGylated compounds124 | |||||
| Half-life (patients >12 y), h | 19.04125 | 14.3-16126 | ∼18.2-19.5127 | ∼19114,116 | — |
| Percentage increase in half-life vs rFVIII | 60%125 § | 40%-50%126 | 28.1%-40.5%127 | 53%-70%114,116 | — |
| Clearance, mL/h/kg | 1.79125 | — | — | 1.68 33%-35% decrease vs rFVIII114 | — |
| Characteristic . | PEGylated FVIII . | Fc fusion platform . | Novel FVIII design . | ||
|---|---|---|---|---|---|
| N8-GP . | BAX 855* . | BAY 94-9027 . | rFVIIIFc† . | rVIII-SingleChain . | |
| Product description | B-domain–modified 40K O-glycoPEGylated rFVIII concentrate | 20-kDa PEGylated full-length rFVIII | BDD rFVIII with a site-specific branched 60-kDa PEG side chain | Recombinant BDD FVIII-Fc fusion protein | Recombinant single-chain FVIII construct |
| Spec.Act. 11 200 IU/mg‡ | Spec.Act. 8000 IU/mg‡ | Spec.Act. 9717 IU/mg‡ | Spec.Act. 9348 IU/mg‡ | Spec.Act. 12 000 IU/mg‡ | |
| Advantages of technology | • Well-established technology | • Established technology | • Increases the intrinsic stability of the FVIII molecule by reducing the potential dissociation of the heavy and light chains | ||
| • Improves solubility of proteins | • Extends half-life of proteins | • Increased affinity for and binding to VWF | |||
| • Extends half-life of non-PEG protein/drug | • May mitigate immunogenicity of the protein | ||||
| • Reduced kidney clearance because of larger hydrodynamic size | |||||
| • Protects the drug from proteolysis | |||||
| • May protect the drug from the immune system | |||||
| • Reduces aggregation | |||||
| Disadvantages of technology | • Random PEGylation (BAX 855) may result in loss or change in protein activity | • Potential for activation of the immune system (antibody-dependent or complement-dependent) | • Not specifically designed for extension of half-life | ||
| • Preexisting anti-PEG antibodies identified in the healthy population, which may result in the rapid clearance of PEGylated compounds124 | |||||
| Half-life (patients >12 y), h | 19.04125 | 14.3-16126 | ∼18.2-19.5127 | ∼19114,116 | — |
| Percentage increase in half-life vs rFVIII | 60%125 § | 40%-50%126 | 28.1%-40.5%127 | 53%-70%114,116 | — |
| Clearance, mL/h/kg | 1.79125 | — | — | 1.68 33%-35% decrease vs rFVIII114 | — |
—, not available; BDD, B-domain deleted.
Adynovate (Baxalta US Inc), approved by the US Food and Drug Administration (FDA) in November 2015.
Eloctate (Biogen Idec Inc), approved by the FDA in June 2014.
Specific activities (Sp.Act.) have been derived using either chromogenic or 1-stage functional assays.
Versus patients’ previous treatment of plasma-derived FVIII or rFVIII.
PEGylation/GlycoPEGylation
PEGylation is being used to prolong the half-lives of many coagulation factors, and there are 3 PEGylated FVIII concentrates currently in development or recently approved: N8-GP, BAX 855 (Adynovate; Baxalta US Inc, Westlake Village, CA), and BAY 94-9027 (Table 1). Polyethylene glycol (PEG) can be selectively added to increase half-life by shielding therapeutic proteins from proteolytic enzymes, clearance receptors, and immune effector cells. Preclinical data in 2 animal models demonstrated that the FVIII-VWF interaction contributes to the longer half-life of PEGylated FVIII.112 The PEGylated FVIII products in development have half-lives that are all ∼1.5-fold longer than that of non-PEGylated FVIII in patients with hemophilia A (Table 3).
Fc fusion proteins
Another technique to increase half-lives of proteins and decrease potential immunogenicity is fusion of the therapeutic protein to a human immunoglobulin Fc fragment. The half-life is thereby extended through Fc interaction with the salvage neonatal Fc receptor.113 A recombinant Fc-FVIII construct (rFVIIIFc; Eloctate, Biogen Inc, Cambridge, MA) has been developed for the treatment of HA (Table 3). Clearance of rFVIIIFc was reduced, resulting in an extended half-life (∼1.5-fold) compared with rFVIII (Table 3).114-116 Furthermore, rFVIIIFc maintains normal FVIII interactions with other proteins necessary for its activity and prolonged in vivo half-life.117 A strong correlation was observed between VWF levels and rFVIIIFc clearance.114,115 For both PEGylated rFVIII and rFVIIIFc, clearance was decreased and half-life was prolonged as endogenous VWF levels increased. Thus, for both PEGylated and Fc-fusion forms of FVIII, clearance and half-life are still modulated by interactions with VWF, thereby limiting the degree to which these strategies can extend the FVIII half-life.
Single-chain design
The heterodimeric 2-chain form of FVIII is the physiologic configuration, but this is a labile structure that can dissociate spontaneously. Thus, a covalently linked single-chain version of FVIII (rVIII-SingleChain) was developed to improve the protein’s stability.
A clinical program for rVIII-SingleChain is under way, but prior preclinical studies have shown improved pharmacodynamic efficacy comparable to full-length and BDD human rFVIII.118 An unexpected observation was that the half-life of rVIII-SingleChain was approximately twofold greater than that of full-length rFVIII.119 This half-life extension may be attributed to the threefold higher affinity of rVIII-SingleChain for plasma-derived VWF compared with full-length rFVIII,120 thus resulting in a smaller proportion of unbound and rapidly cleared rFVIII molecules. This pharmacokinetic behavior has been replicated in a recent phase 1/3 clinical trial and was stable with repeated infusions.121
Future strategies to extend the half-life of FVIII
Despite investigation of numerous approaches, the rFVIII products in late-stage clinical development have achieved only moderate increases in half-life compared with currently marketed rFVIII products (Table 3). The half-life of VWF (∼15 hours) seems to be the limiting factor, suggesting that FVIII may be subject to a dominant VWF-dependent clearance. To overcome the limitation of VWF dependence, 2 novel strategies are currently being investigated. One seeks to prolong the VWF half-life, and the other uses a strategy to limit VWF-dependent clearance of FVIII.
A VWF-albumin fusion protein has been expressed in mammalian cells and preliminary findings show this approach leads to a significantly longer VWF half-life in vivo.120 The second novel strategy to potentially circumvent endogenous VWF-dependent clearance of FVIII is the development of a FVIII molecule comprising 2 polypeptides: a single-chain BDD FVIII with XTEN inserted within the FVIII sequence, and the D’D3 FVIII-binding region of VWF. These polypeptides are fused to the Fc region of immunoglobulin G1 to enable the D′D3 fragment to correctly align to bind the FVIII moiety. This approach has shown favorable pharmacokinetics in animal models, including a fourfold increase in half-life compared with BDD-FVIII.122
Lastly, it should be stated that another novel hemophilia therapeutic agent, emicizumab, a bispecific antibody that possesses FVIII mimetic activity (and is not intended to increase FVIII half-life), appears to have no interaction with VWF, and does not interfere with the treatment of breakthrough bleeding with infused FVIII.123
Conclusions
The critical physiological importance of FVIII and VWF is highlighted by the inherited bleeding disorders HA and VWD. After 35 years of intensive investigation, we are beginning to understand some of the basic biological features of these 2 proteins and their respective and intertwined life cycles. Nevertheless, much still remains to be learned. Why is FVIII expression limited to certain types of endothelium? How are the 2 proteins cleared from plasma? Is there a role for VWF in modulating FVIII immunogenicity? These are just 3 of many unanswered questions. Solving these unknowns will not only advance our basic knowledge of hemostasis physiology, but may also provide enhanced opportunities to treat patients with HA and VWD.
Acknowledgments
D.L. is the recipient of research funding relating to FVIII and VWF from the Canadian Institutes of Health Research and holds a Canada Research Chair in Molecular Hemostasis. P.J.L. is a recipient of a grant from the Agence Nationale de la Recherche (ANR-13-BSV1-0014). K.P.P. acknowledges startup funding from Uniformed Services University of the Health Sciences.
This review was initiated at a face-to-face meeting for which travel support was provided by CSL. CSL did not select the coauthor group and there was no commercial involvement in considering the content or in the writing of this review.
Authorship
Contribution: S.W.P., R.R.M., K.P.P., P.J.L., and D.L. wrote the manuscript and edited versions of the document.
Conflict-of-interest disclosure: S.W.P. has served as a consultant to Baxalta, CSL, Biogen, Novo Nordisk, Roche/Genentech, and Bayer. D.L. receives research support from Biogen, Bayer, CSL, Octapharma, and Sangamo. The remaining authors declare no competing financial interests.
Correspondence: David Lillicrap, Department of Pathology and Molecular Medicine, Richardson Laboratory, Queens University, Kingston, ON, Canada; e-mail: dpl@queensu.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal