Key Points
REP is an active combination in MM patients refractory to lenalidomide.
REP is an all-oral and generally well-tolerated regimen.
Abstract
The prognosis of multiple myeloma (MM) patients who become refractory to lenalidomide and bortezomib is very poor, indicating the need for new therapeutic strategies for these patients. Next to the development of new drugs, the strategy of combining agents with synergistic activity may also result in clinical benefit for patients with advanced myeloma. We have previously shown in a retrospective analysis that lenalidomide combined with continuous low-dose cyclophosphamide and prednisone (REP) had remarkable activity in heavily pretreated, lenalidomide-refractory MM patients. To evaluate this combination prospectively, we initiated a phase 1/2 study to determine the optimal dose and to assess its efficacy and safety in lenalidomide-refractory MM patients. The maximum tolerated dose (MTD) was defined as 25 mg lenalidomide (days 1-21/28 days), combined with continuous cyclophosphamide (50 mg/d) and prednisone (20 mg/d). At the MTD (n = 67 patients), the overall response rate was 67%, and at least minimal response was achieved in 83% of the patients. Median progression-free survival and overall survival were 12.1 and 29.0 months, respectively. Similar results were achieved in the subset of patients with lenalidomide- and bortezomib-refractory disease as well as in patients with high-risk cytogenetic abnormalities, defined as t(4;14), t(14;16), del(17p), and/or ampl(1q) as assessed by fluorescence in situ hybridization. Neutropenia (22%) and thrombocytopenia (22%) were the most common grade 3-4 hematologic adverse events. Infections (21%) were the most common grade 3-5 nonhematologic adverse events. In conclusion, the addition of continuous low-dose oral cyclophosphamide to lenalidomide and prednisone offers a new therapeutic perspective for multidrug refractory MM patients. This trial was registered at www.clinicaltrials.gov as #NCT01352338.
Introduction
The introduction of immunomodulatory drugs (IMiDs), such as thalidomide and lenalidomide, and the proteasome inhibitor, bortezomib, has considerably improved survival of multiple myeloma (MM) patients.1,2 However, the prognosis of patients who become refractory to lenalidomide and bortezomib is very poor, with a median event-free survival of 5 months and overall survival (OS) of 9 months.3 These survival data clearly demonstrate that there is an urgent need for effective, well-tolerated therapies for this category of patients.
In this respect, several new antimyeloma agents have shown activity, including next generation IMiDs (pomalidomide) and proteasome inhibitors (carfilzomib), but also compounds with different mechanisms of action such as histone deacetylase inhibitors, kinesin spindle protein inhibitors, and monoclonal antibodies.4 Next to the development of new drugs, the strategy of combining drugs with synergistic activity may also result in significant clinical benefit for patients with advanced myeloma. Importantly, several large randomized studies comparing lenalidomide-dexamethasone with or without a new agent (carfilzomib, ixazomib, elotuzumab, or daratumumab) have recently shown improved response rates and prolonged PFS in favor of the triplet regimens in MM patients who had received 1 to 3 prior treatments.5-8 However, patients with lenalidomide-refractory disease were not eligible to participate in these clinical trials. In addition, patients with bortezomib-refractory disease were excluded from the studies evaluating the combination of lenalidomide-dexamethasone with ixazomib7 or carfilzomib.8
Lenalidomide has multiple effects in MM, including direct antitumor activity, inhibition of adhesion of MM cells to stromal cells, and suppression of angiogenesis.9 IMiDs also stimulate antitumor response of the immune system through promotion of T-cell costimulation and increase in natural killer cell numbers and activation status.10-12 Similarly, administration of cyclophosphamide, at a dose substantially lower than the maximum tolerated dose (MTD) (metronomic dosing),13 has next to its direct antitumor activity several effects on the bone marrow microenvironment, including immune stimulatory activity.14-22 We hypothesized that the addition of low-dose metronomic oral cyclophosphamide to lenalidomide may be an attractive strategy for lenalidomide-refractory MM patients. Indeed, we previously showed in a small retrospective study that lenalidomide (Revlimid) combined with continuous low-dose oral cyclophosphamide (Endoxan) and prednisone (REP) has remarkable activity in heavily pretreated, lenalidomide-refractory MM patients.23 To assess the optimal dose of this combination and to further evaluate the safety and efficacy of this combination, we initiated a prospective phase 1/2 study in lenalidomide-refractory MM patients.
Here we report the MTD as well as safety and efficacy data from the phase 1/2 REPEAT study.
Materials and methods
Study design
This study was a prospective, investigator-initiated, nonrandomized, multicenter, open-label, phase 1 dose-finding trial (5 dose levels as indicated in supplemental Table 1, available on the Blood Web site), followed by a phase 2 expansion at the recommended dose level (RDL) to evaluate the safety, tolerability, and efficacy of REP in lenalidomide-refractory MM patients (REPEAT study). This study enrolled a total of 82 patients (21 in phase 1 and 61 in phase 2) from August 2011 to November 2014. The dose escalation phase 1 study determined the MTD and RDL of lenalidomide combined with cyclophosphamide and prednisone. The MTD was the RDL for the patients treated in the phase 2 part of the REPEAT study. This trial was conducted in 10 hospitals in the Netherlands. The REPEAT study was approved by the institutional medical ethical committee in each participating center in accordance with the declaration of Helsinki. All participants provided written informed consent. The trial was registered at www.clinicaltrials.gov as #NCT01352338.
Study objectives
The primary objective of the phase 1 study was to identify the MTD and RDL of lenalidomide in combination with cyclophosphamide and prednisone in patients with lenalidomide-refractory MM. The other primary objective of the study was to evaluate the overall response rate (ORR; ≥ partial response [PR]) of REP in patients treated at the MTD. Secondary objectives of the study were to evaluate the clinical benefit rate (≥ minimal response [MR]), to evaluate the safety of the combination, and to assess progression-free survival (PFS) and OS of patients treated at the MTD.
Study population
Patients were eligible to participate in the study if they had lenalidomide-refractory MM following at least 1 prior therapy. Lenalidomide-refractory MM was defined as progressive disease during therapy, no response (< PR) to prior lenalidomide-containing therapy, or within 60 days of discontinuation from lenalidomide-containing regimens, according to the International Myeloma Working Group criteria.3 Similar criteria were used for the definition of bortezomib-refractory myeloma.3 Patients were required to have measurable disease, defined by conventional criteria, as any of the following: (1) serum monoclonal protein ≥10 g/L, (2) urine M-protein ≥200 mg/24 hours, or (3) serum immunoglobulin free light chain ≥ 100 mg/L and abnormal serum immunoglobulin κ to λ free light-chain ratio. Furthermore, a World Health Organization (WHO) performance status of 0 to 3, a platelet count of ≥75 × 109/L, an absolute neutrophil count of ≥1.0 × 109/L, and serum hepatic aminotransferases and bilirubin levels <threefold the upper limit of normal were required. Patients were required to have an estimated creatinine clearance of ≥50 mL/min (Cockcroft-Gault calculation) in phase 1 and ≥30 mL/min in phase 2. Patients had to agree to use contraception in this trial. Exclusion criteria included clinically relevant active comorbid medical or psychiatric conditions or a history of malignancy within the last 5 years.
Drug administration
All drugs were orally administered. Lenalidomide was used on days 1 to 21 of a 28-day cycle, and cyclophosphamide and prednisone were given continuously. REP therapy was given until progression of disease. All patients received thrombosis prophylaxis, consisting of daily aspirin (80 mg), or in patients with a prior history of venous thromboembolism, low-molecular-weight heparin. As infection prophylaxis, patients received cotrimoxazol (480 mg once daily). Patients in dose level 5 of the phase 1 study also received pegylated granulocyte colony-stimulating factor on day 1 of each REP-cycle (supplemental Table 1).
Dose limiting toxicity assessment
See supplemental Methods.
Dose modification
See supplemental Methods.
Safety and efficacy assessments
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).24 Only adverse events of common terminology criteria grade 2 or higher were assessed during each cycle. Safety assessments were done throughout the study, from inclusion until 30 days after the administration of the last dose of any study drug. Treatment response was assessed at the end of each cycle according to the International Myeloma Working Group Uniform Response Criteria,25 with minimal response defined according to European Society for Blood and Marrow Transplantation criteria.26,27
Response was also separately evaluated in patients with high-risk cytogenetic abnormalities as defined by the presence of t(4;14), t(14;16), del(17p), and/or ampl(1q) as determined by fluorescence in situ hybridization (FISH) on purified MM cells before start of REP treatment. Similarly, response was also assessed in the subset of patients with bortezomib- and lenalidomide-double refractory MM.
Statistics
The phase 2 part was designed to determine whether treatment with REP at the MTD warranted further investigation in clinical trials. In order to reject the null hypothesis (ORR: 15%) in favor of the alternative hypothesis (ORR: 30%) with power 1 − β = 0.80 (2-sided significance level, α = 0.05), 53 eligible patients were required. However, in order to overcome dropout, 60 patients were included in the phase 2 part of the trial.
PFS was calculated from day 1 of treatment until progression or death, whichever came first. OS was measured from day 1 of treatment until death from any cause. Patients still alive at the date of last contact were censored. PFS and OS were estimated using the Kaplan-Meier method. Differences between survival curves were tested for statistical significance using the 2-sided log-rank test. Predictive factors for response were determined with the Fisher’s exact test in case of categorical variables and with the Mann-Whitney U test for continuous variables. Univariate Cox regression was used to determine prognostic factors for OS and PFS. Unless otherwise specified, the analyses included either the 21 patients treated in phase 1 or the 67 patients treated at the MTD (dose level 4 phase 1 [n = 6] and phase 2 [n = 61]). All statistical analyses were performed using SPSS software (version 21.0) or graphpad version 6.0. Data were monitored by an external contract research organization (Julius Clinical).
Results
Patient characteristics
A total of 82 patients were enrolled in this phase 1/2 study. Twenty-one patients were enrolled in the phase 1 study, and 61 were enrolled in the phase 2 study. Patient characteristics are shown in Table 1. The median age was 66 years (range, 41-82 years). Median number of prior therapies was 3 (range, 1-10 treatments). All 82 patients had lenalidomide-refractory disease: 66 of these patients (80%) had progressive disease during lenalidomide-based therapy; 11 patients (14%) had progression within 60 days after stopping lenalidomide-containing therapy, and 5 patients (6%) had no response to lenalidomide-containing therapy (see Table 1 for additional details on type of lenalidomide-containing therapy). Seventy-one patients (87%) were also exposed to bortezomib, including 54 (66%) with bortezomib-refractory disease. Forty-seven (57%) patients had previously received cyclophosphamide. High-dose melphalan with autologous stem cell rescue was applied in 50 patients (61%), and allogeneic stem cell transplantation was applied in 8 (10%). Median time from diagnosis to study entry was 48 months (range, 5-169 months). FISH analysis on purified MM cells was performed before the start of REP in 62 of 82 patients (76%); 32 of these 62 patients (52%) were classified as high risk (presence of t(4;14), t(14;16), del(17p), and/or ampl(1q)).
Patient characteristics phase 1/2 REPEAT study
| Characteristic . | Phase 1 (n = 21) . | Phase 2 (n = 61) . | Total (n = 82) . |
|---|---|---|---|
| Median age, y (range) | 69 (41-76) | 65 (43-82) | 66 (41-82) |
| Sex, male, n (%) | 16 (76) | 42 (69) | 58 (71) |
| Type of monoclonal heavy chain, n (%) | |||
| IgG | 11 (52) | 32 (52) | 43 (52) |
| IgA | 6 (29) | 8 (13) | 14 (17) |
| IgD | 0 (0) | 1 (2) | 1 (1) |
| Light chain only | 4 (19) | 20 (33) | 24 (29) |
| Type of light chain, n (%) | |||
| Kappa | 15 (71) | 39 (64) | 54 (66) |
| Lambda | 6 (29) | 22 (36) | 28 (34) |
| Median time from diagnosis until enrollment in months (range) | 41 (18-96) | 51 (5-169) | 48 (5-169) |
| Prior lines of therapy, median (range) | 3 (2-10) | 3 (1-6) | 3 (1-10) |
| Prior therapies, n (%) | |||
| Lenalidomide | 21 (100) | 67 (100) | 82 (100) |
| Bortezomib | 19 (90) | 52 (85) | 71 (87) |
| Thalidomide | 16 (76) | 36 (59) | 52 (63) |
| Cyclophosphamide | 10 (48) | 37 (61) | 47 (57) |
| Prior autologous stem cell transplantation (HDM) | 13 (62) | 37 (61) | 50 (61) |
| Oral melphalan | 11 (52) | 30 (49) | 41 (50) |
| Prior allogeneic stem cell transplantation | 3 (14) | 5 (8) | 8 (10) |
| Previous lenalidomide, n (%) | |||
| Refractory* | 21 (100) | 61 (100) | 82 (100) |
| Progression while on lenalidomide-containing therapy† | 19 (90) | 47 (77) | 66 (80) |
| No response during prior lenalidomide-based therapy‡ | 1 (5) | 4 (7) | 5 (6) |
| Progressive disease within 60 d after stopping lenalidomide-based therapy§ | 1 (5) | 10 (16) | 11 (14) |
| REP directly after development of lenalidomide-refractory disease | 13 (62) | 53 (87) | 66 (80) |
| Lenalidomide and bortezomib double refractory* | 16 (76) | 38 (62) | 54 (66) |
| International Staging System before start REP, n (%) | |||
| 1 | 7 (33) | 15 (27) | 22 (29) |
| 2 | 9 (43) | 25 (46) | 34 (45) |
| 3 | 5 (24) | 15 (27) | 20 (26) |
| WHO Performance Status, n (%) | |||
| 0 | 0 (0) | 10 (17) | 10 (12) |
| 1 | 15 (71) | 33 (56) | 48 (60) |
| 2 | 4 (19) | 11 (19) | 15 (19) |
| 3 | 2 (10) | 5 (8) | 7 (9) |
| β2-Microglobulin median, nmol/L (range) | 3.4 (1.7-10) | 3.4 (0.2-19.1) | 3.4 (0.2-19.1) |
| Laboratory values at baseline, median (range) | |||
| Absolute neutrophil count, ×109/L | 3.2 (1.2-20.5) | 2.6 (1.1-7.9) | 2.6 (1.1-20.5) |
| Hemoglobin, mM | 6.6 (5.3-9.2) | 6.9 (4.5-9.1) | 6.9 (4.5-9.2) |
| Platelet count, ×109/L | 183 (95-334) | 164 (50-369) | 167 (50-369) |
| Creatinine, μmol/L | 86 (58-117) | 86 (53-201) | 86 (53-201) |
| Calcium, mmol/L | 2.35 (2.15-2.64) | 2.31 (1.98-3.35) | 2.31 (1.98-3.35) |
| Cytogenetic abnormalities, n (%) | |||
| High risk|| | 10 (48) | 22 (36) | 32 (39) |
| Standard risk | 10 (48) | 20 (33) | 30 (37) |
| Not available | 1 (4) | 19 (31) | 20 (24) |
| Characteristic . | Phase 1 (n = 21) . | Phase 2 (n = 61) . | Total (n = 82) . |
|---|---|---|---|
| Median age, y (range) | 69 (41-76) | 65 (43-82) | 66 (41-82) |
| Sex, male, n (%) | 16 (76) | 42 (69) | 58 (71) |
| Type of monoclonal heavy chain, n (%) | |||
| IgG | 11 (52) | 32 (52) | 43 (52) |
| IgA | 6 (29) | 8 (13) | 14 (17) |
| IgD | 0 (0) | 1 (2) | 1 (1) |
| Light chain only | 4 (19) | 20 (33) | 24 (29) |
| Type of light chain, n (%) | |||
| Kappa | 15 (71) | 39 (64) | 54 (66) |
| Lambda | 6 (29) | 22 (36) | 28 (34) |
| Median time from diagnosis until enrollment in months (range) | 41 (18-96) | 51 (5-169) | 48 (5-169) |
| Prior lines of therapy, median (range) | 3 (2-10) | 3 (1-6) | 3 (1-10) |
| Prior therapies, n (%) | |||
| Lenalidomide | 21 (100) | 67 (100) | 82 (100) |
| Bortezomib | 19 (90) | 52 (85) | 71 (87) |
| Thalidomide | 16 (76) | 36 (59) | 52 (63) |
| Cyclophosphamide | 10 (48) | 37 (61) | 47 (57) |
| Prior autologous stem cell transplantation (HDM) | 13 (62) | 37 (61) | 50 (61) |
| Oral melphalan | 11 (52) | 30 (49) | 41 (50) |
| Prior allogeneic stem cell transplantation | 3 (14) | 5 (8) | 8 (10) |
| Previous lenalidomide, n (%) | |||
| Refractory* | 21 (100) | 61 (100) | 82 (100) |
| Progression while on lenalidomide-containing therapy† | 19 (90) | 47 (77) | 66 (80) |
| No response during prior lenalidomide-based therapy‡ | 1 (5) | 4 (7) | 5 (6) |
| Progressive disease within 60 d after stopping lenalidomide-based therapy§ | 1 (5) | 10 (16) | 11 (14) |
| REP directly after development of lenalidomide-refractory disease | 13 (62) | 53 (87) | 66 (80) |
| Lenalidomide and bortezomib double refractory* | 16 (76) | 38 (62) | 54 (66) |
| International Staging System before start REP, n (%) | |||
| 1 | 7 (33) | 15 (27) | 22 (29) |
| 2 | 9 (43) | 25 (46) | 34 (45) |
| 3 | 5 (24) | 15 (27) | 20 (26) |
| WHO Performance Status, n (%) | |||
| 0 | 0 (0) | 10 (17) | 10 (12) |
| 1 | 15 (71) | 33 (56) | 48 (60) |
| 2 | 4 (19) | 11 (19) | 15 (19) |
| 3 | 2 (10) | 5 (8) | 7 (9) |
| β2-Microglobulin median, nmol/L (range) | 3.4 (1.7-10) | 3.4 (0.2-19.1) | 3.4 (0.2-19.1) |
| Laboratory values at baseline, median (range) | |||
| Absolute neutrophil count, ×109/L | 3.2 (1.2-20.5) | 2.6 (1.1-7.9) | 2.6 (1.1-20.5) |
| Hemoglobin, mM | 6.6 (5.3-9.2) | 6.9 (4.5-9.1) | 6.9 (4.5-9.2) |
| Platelet count, ×109/L | 183 (95-334) | 164 (50-369) | 167 (50-369) |
| Creatinine, μmol/L | 86 (58-117) | 86 (53-201) | 86 (53-201) |
| Calcium, mmol/L | 2.35 (2.15-2.64) | 2.31 (1.98-3.35) | 2.31 (1.98-3.35) |
| Cytogenetic abnormalities, n (%) | |||
| High risk|| | 10 (48) | 22 (36) | 32 (39) |
| Standard risk | 10 (48) | 20 (33) | 30 (37) |
| Not available | 1 (4) | 19 (31) | 20 (24) |
HDM, high-dose melphalan.
Refractory disease is defined as progressive disease during therapy, no response (< PR), or progressive disease within 60 d of stopping treatment, according to the International Uniform Response Criteria for Multiple Myeloma.
Fifty patients progressed while receiving lenalidomide (25 mg)-dexamethasone, 6 while receiving lenalidomide, bortezomib, and dexamethasone, and 10 while receiving lenalidomide maintenance therapy (10 mg).
Three patients received lenalidomide (25 mg)-dexamethasone; 1 patient received 10 mg lenalidomide in MPR (melphalan, prednisone, lenalidomide), and 1 patient received lenalidomide (10 mg) maintenance therapy.
Ten patients received lenalidomide (25 mg)-dexamethasone, and 1 patient received 10 mg lenalidomide in MPR.
High-risk cytogenetic abnormalities were defined by the presence of t(4;14), t(14;16), del(17p), and/or ampl(1q) as determined by FISH analysis on purified MM cells before start of REP treatment. FISH analysis on purified MM cells was performed before the start of REP in 62 of 82 patients.
Maximum tolerated dose
We first evaluated the combination of lenalidomide, cyclophosphamide, and prednisone in the dose-finding, phase 1 part of the study, in which 21 lenalidomide-refractory patients were treated at 5 different dose levels (supplemental Table 1). The MTD was determined to be dose level 4 with 25 mg lenalidomide on days 1 to 21, combined with continuous cyclophosphamide (50 mg/d) and prednisone (20 mg/d). Details on the phase 1 part of the study are given in the supplements (including supplemental Tables 2 and 3).
Safety of REP at the MTD
Sixty-one additional patients were subsequently treated at the MTD in the phase 2 part of the study to further assess the safety and activity profile of REP. In the safety analysis, we also included the 6 patients treated at dose level 4 (MTD dose level) in the phase 1 part of the study (total of 67 patients).
All 67 patients could be evaluated for hematologic and nonhematologic adverse events, which were assessed from the start of REP treatment until 1 month after stopping therapy (Table 2). The most frequent adverse events in patients treated at the MTD were hematologic toxicities with grade 3 neutropenia in 13 patients (19%) and grade 4 neutropenia in 2 patients (3%). These patients were successfully treated with granulocyte colony-stimulating factor in subsequent cycles without further interruptions of therapy. Grade 3 and 4 anemia occurred in 3 (4%) and 0 (0%) patients, respectively. Grade 3 and 4 thrombocytopenia occurred in 10 (15%) and 5 (7%) patients, respectively. These toxicities were well managed with dose delays or dose reductions. The most common nonhematologic toxicities were infections: 18% of the patients experienced a grade 3 infection during REP treatment (mostly upper and lower respiratory tract infections), and 2 (3%) patients succumbed to pneumonia with septic shock. Cardiac disorders developed in 5 patients: 1 patient had grade 3 angina pectoris caused by anemia, which recovered completely after blood transfusion; 1 patient experienced palpitations caused by self-limiting unexplained ventricular arrhythmia; and 3 patients experienced heart failure (grade 3 in 2 patients and grade 4 in 1 patient). Two of these 3 patients with heart failure had a history of cardiac disease. Grade 3 venous thromboembolism was reported in 3 patients: 2 patients with pulmonary embolism, despite low-molecular-weight heparin administered because of a history of previous pulmonary embolism, and 1 patient had a deep venous thrombosis, despite prophylactic therapy with aspirin. Treatment-emergent peripheral neuropathy was uncommon, with 4 patients experiencing grade 2 peripheral neuropathy. None of the patients developed a second primary malignancy (SPM). Furthermore, none of the patients who previously underwent allogeneic stem cell transplantation developed graft-versus-host disease during REP treatment. During the course of the study, toxicity led to at least 1 level of dose reduction for lenalidomide in 11 patients (16%), whereas there were no dose reductions for cyclophosphamide or prednisone. Eight patients (12%) discontinued therapy because of adverse events.
Adverse events for patients treated at the MTD (dose level 4 of phase 1 and phase 2)
| n = 67 . | |||||
|---|---|---|---|---|---|
| Events . | Grade 2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . | Grade 5, n (%) . | Total, n (%) . |
| Hematologic | |||||
| Neutropenia | 8 (12) | 13 (19) | 2 (3) | — | 23 (34) |
| Thrombocytopenia | 10 (15) | 10 (15) | 5 (7) | — | 25 (37) |
| Anemia | 4 (6) | 3 (4) | — | — | 7 (10) |
| Nonhematologic | |||||
| Thromboembolism | — | 3 (4) | — | — | 3 (4) |
| Constitutional | 21 (31) | 2 (3) | — | — | 23 (34) |
| Fatigue | 9 (13) | 1 (1) | — | — | 10 (15) |
| Muscle cramps | 12 (18) | 1 (1) | — | — | 13 (19) |
| Neurologic | 4 (6) | 1 (1) | — | — | 5 (8) |
| Sensory neuropathy | 4 (6) | — | — | — | 4 (6) |
| Dysesthesia | — | 1 (1) | — | — | 1 (1) |
| Infections | 25 (37) | 12 (18) | — | 2 (3) | 39 (58) |
| Upper respiratory | 12 (18) | 4 (7) | — | — | 16 (24) |
| Pneumonia | 2 (3) | 6 (9) | — | 2 (3) | 10 (15) |
| Gastrointestinal | 5 (7) | 1 (1) | 6 (9) | ||
| Herpes zoster | 2 (3) | — | — | 2 (3) | |
| Other | 4 (7) | 1 (1) | — | — | 5 (7) |
| Cardiac disorders | — | 4 (7) | 1 (1) | — | 5 (7) |
| Congestive heart failure | — | 2 (3) | 1 (1) | — | 3 (4) |
| Arythmia | — | 1 (1) | — | — | 1 (1) |
| Angina pectoris | — | 1 (1) | — | — | 1 (1) |
| n = 67 . | |||||
|---|---|---|---|---|---|
| Events . | Grade 2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . | Grade 5, n (%) . | Total, n (%) . |
| Hematologic | |||||
| Neutropenia | 8 (12) | 13 (19) | 2 (3) | — | 23 (34) |
| Thrombocytopenia | 10 (15) | 10 (15) | 5 (7) | — | 25 (37) |
| Anemia | 4 (6) | 3 (4) | — | — | 7 (10) |
| Nonhematologic | |||||
| Thromboembolism | — | 3 (4) | — | — | 3 (4) |
| Constitutional | 21 (31) | 2 (3) | — | — | 23 (34) |
| Fatigue | 9 (13) | 1 (1) | — | — | 10 (15) |
| Muscle cramps | 12 (18) | 1 (1) | — | — | 13 (19) |
| Neurologic | 4 (6) | 1 (1) | — | — | 5 (8) |
| Sensory neuropathy | 4 (6) | — | — | — | 4 (6) |
| Dysesthesia | — | 1 (1) | — | — | 1 (1) |
| Infections | 25 (37) | 12 (18) | — | 2 (3) | 39 (58) |
| Upper respiratory | 12 (18) | 4 (7) | — | — | 16 (24) |
| Pneumonia | 2 (3) | 6 (9) | — | 2 (3) | 10 (15) |
| Gastrointestinal | 5 (7) | 1 (1) | 6 (9) | ||
| Herpes zoster | 2 (3) | — | — | 2 (3) | |
| Other | 4 (7) | 1 (1) | — | — | 5 (7) |
| Cardiac disorders | — | 4 (7) | 1 (1) | — | 5 (7) |
| Congestive heart failure | — | 2 (3) | 1 (1) | — | 3 (4) |
| Arythmia | — | 1 (1) | — | — | 1 (1) |
| Angina pectoris | — | 1 (1) | — | — | 1 (1) |
Efficacy of REP at the MTD
Sixty-six of 67 patients treated at the MTD were evaluable for response; in 1 patient, no response evaluation was performed during 2 courses of REP, after which treatment was stopped because of grade 3 fatigue, without signs of progression. Patients received a median of 9 REP cycles (range 1-30+ cycles). The ORR (≥ PR) was 67% (44 patients), including at least very good partial response (VGPR) in 15 patients (23%). Three patients achieved complete remission (CR) (5%), including 1 stringent CR (sCR). Eleven patients (16%) achieved an MR, translating to an overall 83% clinical benefit rate (≥ MR). At least stable disease was achieved in 60 patients (91%) (Table 3). Median time to at least PR was 1.7 months (range, 0.5-22.8 months). Response to REP was better in 25%, similar in 44%, and inferior in 31% of patients, when compared with the preceding lenalidomide-containing regimen. Two of the 3 patients who achieved (s)CR during REP had also obtained CR in the preceding lenalidomide-containing regimen, whereas the other patient had a VGPR.
Response of patients treated at the MTD (dose level 4 of phase 1 and phase 2)
| . | All patients (all len-refractory), n = 66, % . | Len- and bor-refractory patients, n = 42, % . | Patients with high-risk cytogenetic abnormalities,* n = 24, % . | Patients treated with REP, directly following development of len-refractory disease (25 mg len or equivalent in case of renal insufficiency), n = 46, % . |
|---|---|---|---|---|
| sCR | 1.5 | 0 | 0 | 0 |
| CR | 3.0 | 2.4 | 0 | 0 |
| VGPR | 18.2 | 21.4 | 20.8 | 15.2 |
| PR | 44.0 | 36.1 | 45.9 | 50.0 |
| MR | 16.6 | 21.1 | 16.6 | 17.4 |
| SD | 7.6 | 9.5 | 4.2 | 10.9 |
| PD | 9.1 | 9.5 | 12.5 | 6.5 |
| ≥VGPR | 22.7 | 23.8 | 20.8 | 15.2 |
| ≥PR | 66.7 | 59.9 | 66.7 | 65.2 |
| ≥MR | 83.3 | 81.0 | 83.3 | 82.6 |
| . | All patients (all len-refractory), n = 66, % . | Len- and bor-refractory patients, n = 42, % . | Patients with high-risk cytogenetic abnormalities,* n = 24, % . | Patients treated with REP, directly following development of len-refractory disease (25 mg len or equivalent in case of renal insufficiency), n = 46, % . |
|---|---|---|---|---|
| sCR | 1.5 | 0 | 0 | 0 |
| CR | 3.0 | 2.4 | 0 | 0 |
| VGPR | 18.2 | 21.4 | 20.8 | 15.2 |
| PR | 44.0 | 36.1 | 45.9 | 50.0 |
| MR | 16.6 | 21.1 | 16.6 | 17.4 |
| SD | 7.6 | 9.5 | 4.2 | 10.9 |
| PD | 9.1 | 9.5 | 12.5 | 6.5 |
| ≥VGPR | 22.7 | 23.8 | 20.8 | 15.2 |
| ≥PR | 66.7 | 59.9 | 66.7 | 65.2 |
| ≥MR | 83.3 | 81.0 | 83.3 | 82.6 |
Len, lenalidomide; bor, bortezomib; SD, stable disease; PD, progressive disease.
High-risk disease was defined by the presence of t(4;14), t(14;16), del(17p), and/or ampl(1q) as determined by FISH on purified MM cells before start of REP treatment.
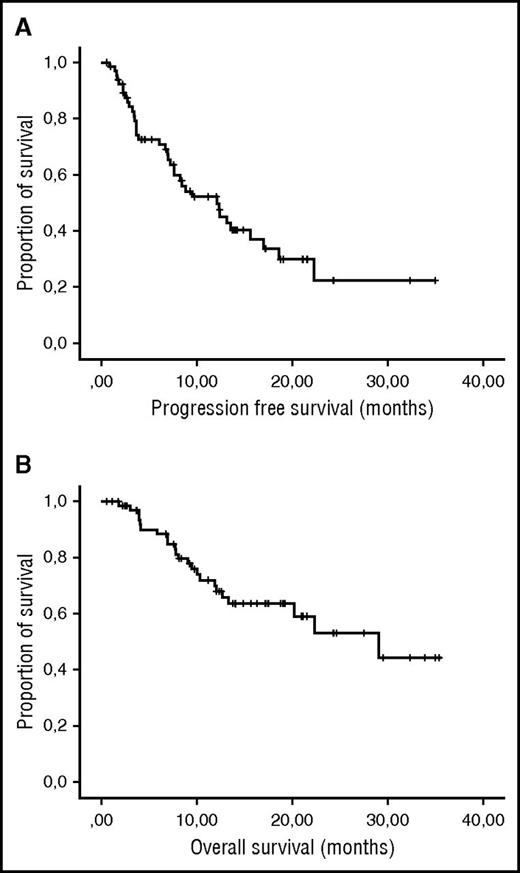
After a median follow-up of 24.5 months (range 1.1-33.9+), the median PFS was 12.1 months and the median OS was 29.0 months (Figure 1). Patients who reached ≥ PR (median PFS: 15.6 months) or ≥ VGPR (median PFS: not reached) had a significantly better PFS than those with responses < PR (median PFS: 3.7 months). OS was also better in patients with PR (median OS: 29.0 months) or VGPR (median OS: 30.9 months) as compared with patients with < PR (median OS: 11.9 months), but this did not reach statistical significance.
PFS and OS for patients treated at the MTD. (A) PFS and (B) OS of the 67 lenalidomide-refractory MM patients treated with REP at the MTD.
PFS and OS for patients treated at the MTD. (A) PFS and (B) OS of the 67 lenalidomide-refractory MM patients treated with REP at the MTD.
Median PFS for the preceding lenalidomide-containing regimen was 11.2 months (median of 2 prior therapies). Supplemental Table 4 shows the median PFS for patients treated with REP and with the preceding lenalidomide-containing regimen for several subgroups.
Prognostic factors for response, PFS, and OS
Forty-seven patients, treated at the MTD, were evaluated for cytogenetic abnormalities by FISH. Twenty-four of these patients (51%) had high-risk cytogenetic abnormalities. Response in these patients was similar to that observed in standard-risk patients (Table 3). Furthermore, PFS and OS did not differ between patients with high risk and standard risk as defined by FISH (median PFS: 12.1 vs 12.3 months, P = .943; median OS: 22.3 vs 29.0 months, P = .982 for high-risk and standard-risk patients, respectively) (Figure 2A,B).
PFS and OS for patients treated at the MTD for different subgroups. (A) PFS and (B) OS for patients treated with REP at the MTD with high-risk disease (presence of t(4;14), t(14;16), del(17p), and/or ampl(1q) as determined by FISH) vs standard-risk disease. (C) PFS and (D) OS for patients treated with REP at the MTD with disease refractory to both lenalidomide and bortezomib (double-refractory MM) vs disease refractory to only lenalidomide. (E) PFS and (F) OS for patients treated with REP at the MTD with REP directly given after development of lenalidomide-refractory disease vs REP given after at least 1 other line of therapy after the development of lenalidomide-refractory disease. (G) PFS and (H) OS for patients treated with REP at the MTD with <3 vs ≥3 prior lines of therapy.
PFS and OS for patients treated at the MTD for different subgroups. (A) PFS and (B) OS for patients treated with REP at the MTD with high-risk disease (presence of t(4;14), t(14;16), del(17p), and/or ampl(1q) as determined by FISH) vs standard-risk disease. (C) PFS and (D) OS for patients treated with REP at the MTD with disease refractory to both lenalidomide and bortezomib (double-refractory MM) vs disease refractory to only lenalidomide. (E) PFS and (F) OS for patients treated with REP at the MTD with REP directly given after development of lenalidomide-refractory disease vs REP given after at least 1 other line of therapy after the development of lenalidomide-refractory disease. (G) PFS and (H) OS for patients treated with REP at the MTD with <3 vs ≥3 prior lines of therapy.
Forty-two patients of the 67 patients treated at the MTD (64%) had disease refractory to both lenalidomide and bortezomib. Also, in this subgroup response, PFS and OS were not statistically different, as compared with patients who were not bortezomib refractory (Figure 2C,D). In addition, patients (n = 46, 67%) who received REP directly after development of lenalidomide-refractory disease had similar response and survival, when compared with patients who received REP after 1 or more other lines of therapy (Table 3; Figure 2E,F).
WHO performance status before the start of REP treatment was the only variable, which was significantly associated with response (Table 4). Patients with a WHO performance status of 2 or 3 had a significantly lower response rate than patients with performance status of 0 or 1. There were no differences in extent of dose reduction of study medication between these 2 groups.
Univariate analysis of possible predictive factors for PFS and OS
| . | ORR . | PFS . | OS . | ||
|---|---|---|---|---|---|
| P . | P . | HR (95% CI) . | P . | HR (95% CI) . | |
| >65 y | .486 | .901 | 1.024 (0.545-1.993) | .81 | 1.105 (0.486-2.510) |
| Male | .705 | .315 | 1.415 (0.719-2.784) | .786 | 1.127 (0.476-2.669) |
| ≥3 lines of therapy | .144 | .830 | 1.079 (0.539-2.157) | .553 | 1.289 (0.557-2.981) |
| ≥4 lines of therapy | .855 | .651 | 0.859 (0.446-1.657) | .214 | 0.595 (0.262-1.351) |
| Creatinine clearance (≥50 mL/min) | .320 | .410 | 0.643 (0.225-1.837) | .418 | 2.296 (0.307-17.163) |
| Thrombocytes | .217 | .911 | 1.0 (0.995-1.005) | .209 | 1.004 (0.998-1.009) |
| β2-microgobulin | .697 | .050 | 1.122 (1.000-1.259) | .369 | 1.058 (0.935-1.197) |
| Albumin | .361 | .253 | 1.001 (0.999-1.003) | .147 | 1.002 (0.999-1.005) |
| LDH | .525 | .081 | 1.002 (1.000-1.004) | .001 | 1.004 (1.001-1.006) |
| High-risk cytogenetics | .705 | .816 | 0.912 (0.420-1.981) | .958 | 0.974 (0.367-2.584) |
| WHO 0+1 vs 2+3 | .010 | .867 | 0.932 (0.405-2.141) | .238 | 0.567 (0.222-1.453) |
| Len-refractory vs len- and bor-refractory disease | .103 | .376 | 0.736 (0.374-1.450) | .798 | 0.896 (0.386-2.078) |
| Full-dose lenalidomide (25 mg or equivalent in case of renal insufficiency) before start REP-therapy | 1.00 | .497 | 1.299 (0.610-2.765) | .950 | 1.031 (0.402-2.643) |
| REP directly after development of len-refractory disease | .861 | .951 | 1.02 (0.532-1.957) | .785 | 1.122 (0.491-2.568) |
| . | ORR . | PFS . | OS . | ||
|---|---|---|---|---|---|
| P . | P . | HR (95% CI) . | P . | HR (95% CI) . | |
| >65 y | .486 | .901 | 1.024 (0.545-1.993) | .81 | 1.105 (0.486-2.510) |
| Male | .705 | .315 | 1.415 (0.719-2.784) | .786 | 1.127 (0.476-2.669) |
| ≥3 lines of therapy | .144 | .830 | 1.079 (0.539-2.157) | .553 | 1.289 (0.557-2.981) |
| ≥4 lines of therapy | .855 | .651 | 0.859 (0.446-1.657) | .214 | 0.595 (0.262-1.351) |
| Creatinine clearance (≥50 mL/min) | .320 | .410 | 0.643 (0.225-1.837) | .418 | 2.296 (0.307-17.163) |
| Thrombocytes | .217 | .911 | 1.0 (0.995-1.005) | .209 | 1.004 (0.998-1.009) |
| β2-microgobulin | .697 | .050 | 1.122 (1.000-1.259) | .369 | 1.058 (0.935-1.197) |
| Albumin | .361 | .253 | 1.001 (0.999-1.003) | .147 | 1.002 (0.999-1.005) |
| LDH | .525 | .081 | 1.002 (1.000-1.004) | .001 | 1.004 (1.001-1.006) |
| High-risk cytogenetics | .705 | .816 | 0.912 (0.420-1.981) | .958 | 0.974 (0.367-2.584) |
| WHO 0+1 vs 2+3 | .010 | .867 | 0.932 (0.405-2.141) | .238 | 0.567 (0.222-1.453) |
| Len-refractory vs len- and bor-refractory disease | .103 | .376 | 0.736 (0.374-1.450) | .798 | 0.896 (0.386-2.078) |
| Full-dose lenalidomide (25 mg or equivalent in case of renal insufficiency) before start REP-therapy | 1.00 | .497 | 1.299 (0.610-2.765) | .950 | 1.031 (0.402-2.643) |
| REP directly after development of len-refractory disease | .861 | .951 | 1.02 (0.532-1.957) | .785 | 1.122 (0.491-2.568) |
CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase.
We also performed univariate Cox regression analysis to determine prognostic factors for PFS and OS. The only variable significantly associated with impaired PFS was an elevated pretreatment β2-microglobulin level. High pretreatment LDH levels were significantly associated with reduced OS, whereas there was a trend toward impaired PFS. All other factors tested, including extent of prior therapy, were not associated with PFS and OS (Table 4; Figure 2G,H).
Discussion
In this phase 1/2 trial, we evaluated the MTD as well as the safety and efficacy of REP in heavily pretreated, lenalidomide-refractory MM patients (66% of the patients were also refractory to bortezomib). The MTD was determined to be 25 mg of lenalidomide on days 1 to 21 of a 28-day cycle, combined with continuous oral cyclophosphamide at a dose of 50 mg, and prednisone at a dose of 20 mg. The REP regimen was well tolerated and highly active with an ORR (≥ PR) in 67% and a clinical benefit rate (≥ MR) in 82%. The median PFS was 12.1 months, and the median OS was 29.0 months.
Hematologic toxicities in our study were acceptable and consistent with the observed toxicities in MM patients treated with lenalidomide-dexamethasone.28,29 Similarly, when cyclophosphamide was added to pomalidomide and prednisone,30 hematologic toxicity was comparable to the toxicity observed with pomalidomide-dexamethasone.31,32 Altogether, this suggests that low-dose cyclophosphamide does not significantly increase hematologic toxicity, when it is added to lenalidomide or pomalidomide. In contrast, melphalan has more profound myelosuppression, making this alkylating agent less attractive to use in combination with lenalidomide.33,34 Nonhematologic toxicity of REP consisted mainly of infections. Discontinuations because of adverse events were uncommon, allowing patients to continue therapy until disease progression. No SPMs were observed in this study. Indeed, several other studies have demonstrated that lenalidomide combined with cyclophosphamide is associated with a markedly lower risk of SPM, when compared with lenalidomide plus melphalan.35,36
We observed high activity of REP despite enrolling patients who were all lenalidomide refractory and 66% who were also bortezomib refractory. Although the importance of high-risk cytogenetic features in advanced relapsed/refractory MM has not been clearly defined, we observed similar response and survival in patients with high-risk cytogenetic abnormalities when compared with standard-risk patients. Outcome was also similar in patients with lenalidomide and bortezomib, double-refractory, MM. Notably, we observed a median PFS of 14.3 months and median OS not yet reached in double-refractory MM patients, which compares favorably with historical controls of patients who were refractory to both IMiDs and bortezomib, who had a median event-free survival of 5 months and median OS of 9 months.3 However, response was inferior in patients with a WHO performance status score of 2 or 3, but this did not translate into reduced PFS or OS. In addition, in our study, elevated β2-microglobulin was predictive of impaired PFS, whereas high LDH levels were associated with shorter OS. These subgroups involve relatively small numbers of patients, and further analysis is needed to assess the impact of these variables on outcome with REP.
We previously showed that the 2-drug combination of continuous low-dose cyclophosphamide and prednisone has also significant anti-MM activity in relapsed/refractory MM patients, who were not previously exposed to novel agents.37 However, another study showed that low-dose cyclophosphamide (50 mg daily) combined with steroids has markedly lower activity in lenalidomide- and bortezomib-exposed patients (63% of these patients were double-refractory to bortezomib and IMiDs), with at least PR in 11.4% of these patients and a median PFS and OS of only 3.3 months and 10.0 months, respectively.38 This outcome is inferior to that observed with the REP regimen in double-refractory MM patients and suggests clinical synergy between lenalidomide and low-dose cyclophosphamide.
The combination of lenalidomide, cyclophosphamide, and steroid has also been studied in newly diagnosed MM.35,39 Kumar et al demonstrated high efficacy of the 3-drug combination with weekly cyclophosphamide (≥ PR: 85%).39 However, lenalidomide combined with cyclophosphamide and prednisone was not superior to lenalidomide-dexamethasone in a phase 3 clinical trial with elderly newly diagnosed MM patients.35 This nonsuperiority may be explained in part by the low dose of lenalidomide (10 mg, days 1-21/28-day cycle) and cyclophosphamide (50 mg every other day), which were increased after protocol amendment.35
Other studies have also demonstrated a beneficial effect of addition of weekly cyclophosphamide to lenalidomide and corticosteroids in patients with relapsed/refractory lenalidomide-naive MM (≥ PR: 65% to 94%).40-42 Because of the high response and prolonged PFS reported in these studies, directly starting with the 3-drug regimen of lenalidomide, cyclophosphamide, and corticosteroid may also be considered, as opposed to adding cyclophosphamide at the time of development of lenalidomide-refractory disease. Furthermore, a retrospective analysis showed high efficacy (≥ PR: 68%) and good tolerability of lenalidomide, low-dose cyclophosphamide, and prednisone in relapsed/refractory MM patients who were previously exposed to lenalidomide-dexamethasone (39% lenalidomide refractory).43 Similarly, it has recently been shown that addition of cyclophosphamide to pomalidomide and dexamethasone in lenalidomide-refractory MM increases the ORR from 39% to 65% and median PFS from 4.4 to 9.5 months.44 Larocca et al also showed that pomalidomide in combination with cyclophosphamide-prednisone is effective and well tolerated in lenalidomide- and bortezomib-refractory MM patients (≥ PR: 50%; median PFS: 8.6 months).30 However, although our data suggest synergy between lenalidomide and cyclophosphamide, a formal comparison between REP and low-dose cyclophosphamide-prednisone alone, would be needed to substantiate our findings.
Importantly, ORRs with REP were higher and median PFS was longer, when compared with the outcome of next generation novel agents evaluated in lenalidomide-refractory MM. Treatment with pomalidomide plus dexamethasone results in at least PR in 31% of patients with a median PFS of 4.0 months (75% lenalidomide- and bortezomib-refractory MM patients).32 Carfilzomib monotherapy induces at least PR in 19.1% of extensively pretreated patients with a median PFS of 3.7 months.38 Daratumumab induces at least PR in 29% to 36% of patients with a median PFS of 3.7 to 5.6 months,45,46 whereas elotuzumab47 has no single-agent activity in this setting. The outcome of the REP regimen also compares favorably to the results of carfilzomib- or pomalidomide-based combinations in relapsed/refractory MM patients (the majority are lenalidomide refractory), such as pomalidomide-bortezomib-dexamethasone (≥ PR: 85%, median PFS 10.7 months),48 pomalidomide-carfilzomib-dexamethasone (≥ PR: 50%, median PFS: 7.2 months),49 and daratumumab-pomalidomide-dexamethasone (≥ PR: 71%, PFS at 6 months: 66%).50 Nevertheless, cross-trial comparisons must be interpreted with caution, because such a comparison might be biased by multiple factors as differences in trial sizes, patient populations, and study designs.
Other lenalidomide-based combinations were also evaluated in lenalidomide-refractory MM. Interestingly, there was synergy between lenalidomide and therapeutic antibodies such as pembrolizumab (anti–programmed cell death protein-1; ≥ PR: 38%)51 and isatuximab (anti-CD38; ≥ PR: 48%)52 in lenalidomide-refractory patients, suggesting that the immune system of these patients could still respond to the immunomodulatory effects of lenalidomide. Similarly, a retrospective study showed that the combination of lenalidomide, bortezomib, and dexamethasone may be effective in heavily pretreated patients (50% lenalidomide-refractory) with an ORR of 47% and median PFS of 3.0 months.53
The efficacy of lenalidomide plus continuous low-dose oral cyclophosphamide in lenalidomide-refractory MM raises questions about mechanisms of action of this regimen. It is well known that metronomic low-dose cyclophosphamide has multiple effects, including direct antitumor activity, antiangiogenic effects,18,19 modulation of the microenvironment,21 and improvement of T and natural killer cell–mediated antitumor immune response via depletion of regulatory T cells.14-17,20,22 Also, lenalidomide has pleiotropic effects on the tumor microenvironment, but via different pathways, which may explain the synergy between these drugs in lenalidomide-refractory patients.9 It is currently unclear whether weekly higher-dose cyclophosphamide has the same effects on the bone marrow microenvironment and the same activity in patients, when compared with continuous low-dose cyclophosphamide. Additional studies are needed to resolve these questions.
Although several of the new agents to treat lenalidomide- and bortezomib-refractory MM are now approved by the US Food and Drug Administration and/or the European Medicines Agency, these therapies may not yet be available or reimbursed in many countries, whereas cyclophosphamide is widely available. In addition, REP is a fully oral 3-drug combination, which is convenient for patients but also likely associated with lower costs of patient care. Altogether, this further highlights the importance of this effective salvage strategy for heavily pretreated relapsed/refractory MM patients.
In summary, REP, at the MTD of lenalidomide (25 mg, days 1-21/28 days), continuous low-dose cyclophosphamide (50 mg) and prednisone (20 mg), is a fully oral, well-tolerated and active combination for patients with lenalidomide- and bortezomib-refractory MM. Therefore, the addition of continuous low-dose oral cyclophosphamide to lenalidomide and prednisone may offer new therapeutic perspectives for multidrug-resistant MM patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contributions: H.M.L., S.Z., and N.W.C.J.v.d.D. designed the study; I.S.N., H.M.L., M.-D.L., G.M.J.B., A. Broijl, A. Beeker, S.K.K., H.R.K., L.M.F., E.v.d.S., P.F.Y., L.E.F., R.R., D.-J.v.S., P.E.W., R.O., P.S., S.Z., and N.W.C.J.v.d.D. treated patients; I.S.N., H.M.L., S.Z., N.W.C.J.v.d.D., A.C.B., B.v.K., and T.M. interpreted the results; I.S.N. and N.W.C.J.v.d.D. wrote the first draft of the manuscript; all authors critically reviewed the manuscript and checked the final version of it.
Conflict-of-interest disclosure: H.M.L., T.M., and N.W.C.J.v.d.D. received research support from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Niels W. C. J. van de Donk, Department of Hematology, VU University Medical Center, De Boelelaan 1117, 1081HV Amsterdam, The Netherlands; e-mail: n.vandedonk@vumc.nl.