In this issue of Blood, Kim et al focused on the potential role of the proinflammatory cytokine interferon-α (IFN-α) in the developmental maturation of aorta-gonad-mesonephros (AGM) region hematopoietic stem cells (HSCs). They find that treatment of AGM HSCs with IFN-α increases long-term hematopoietic engraftment and donor chimerism. In addition, they identify adenine-thymine–rich interactive domain-3a (Arid3a) as an important transcriptional co-regulator of IFN-α signaling in embryonic HSCs.1
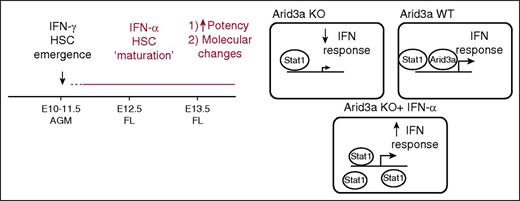
The role of IFN-α during embryonic hematopoiesis. Although IFN-γ has been reported to play a role in promoting emergence of HSCs, Kim et al show that IFN-α promotes the next step in development of HSCs: the maturation of AGM HSCs. In this process, IFN-α signals parallel to Arid3a, with Arid3a being a transcriptional co-regulator of IFN effector genes. Therefore, when Arid3a is absent, IFN signaling in AGM HSCs is dampened. Exogenous IFN-α treatment of AGM HSCs will lead to saturation of the system with STAT1, overcoming this defect. See the complete Figure 7 in the article by Kim et al that begins on page 204.
The role of IFN-α during embryonic hematopoiesis. Although IFN-γ has been reported to play a role in promoting emergence of HSCs, Kim et al show that IFN-α promotes the next step in development of HSCs: the maturation of AGM HSCs. In this process, IFN-α signals parallel to Arid3a, with Arid3a being a transcriptional co-regulator of IFN effector genes. Therefore, when Arid3a is absent, IFN signaling in AGM HSCs is dampened. Exogenous IFN-α treatment of AGM HSCs will lead to saturation of the system with STAT1, overcoming this defect. See the complete Figure 7 in the article by Kim et al that begins on page 204.
Traditionally, it was thought that inflammation and infection played only an indirect role in HSC biology in the bone marrow (BM) of adults. Recently, several studies have investigated the effect of proinflammatory cytokines such as interferons on HSCs and have found that HSCs can directly respond to IFN-α, IFN-β, IFN-γ, and tumor necrosis factor α (TNF-α) in vivo,2-4 resulting in increased proliferation of these cells.
All of these studies focused on the link between inflammatory signaling and HSCs in the adult. It was only recently that focus shifted to the role of proinflammatory cytokines during the development of the hematopoietic system. Studies that used mice and zebrafish have shown that IFN-γ signaling regulates the production of the first hematopoietic stem and progenitor cells in the AGM region in the embryo.5,6 TNF-α signaling was also shown to play an important role in the emergence of the first HSCs in zebrafish.7 These data have uncovered a role for inflammatory signaling in HSC production in the AGM region.
After HSCs arise in the AGM region, they migrate to the fetal liver (FL) before moving to the BM to maintain adult hematopoiesis. While progressing through the different embryonic development stages, HSCs alter molecularly and functionally. AGM HSCs, for example, have reduced repopulation capacity in adult BM transplantation compared with FL HSCs,8 suggesting that AGM HSCs are immature compared with those that have already migrated to the FL. Little is known about the signaling pathways promoting this next step of developmental maturation of AGM HSCs.
Kim et al demonstrated an additional role for IFN signaling in the functional maturation of AGM HSCs. They performed several bioinformatics analyses on their previously published microarray data set of HSCs at different developmental stages9 and predicted that the Jak-Stat signaling pathway, mediated by IFN-Stat1 signaling, would be low in AGM HSCs compared with FL HSCs, thus accounting for key differences between them. In addition, expression-level analysis confirmed higher levels of IFN-α in the FL. However, AGM HSCs did respond to treatment with IFN-α, despite the low levels of Jak-Stat1 signaling. In fact, pretreatment of AGM HSCs with IFN-α promoted the long-term engraftment of these HSCs, which was even more pronounced in secondary transplants. Further analysis that included limiting dilution assays showed that there is no significant difference in frequency or homing capacity of HSCs. The authors attribute the increase in engraftment to an increase in percentage of quiescent HSCs in the recipients, although the mechanism for this remains uncertain.
To find co-regulators of IFN-α during the maturation of AGM HSCs, Kim et al screened the previously published microarray of HSCs treated in vivo2 and focused on Arid3a. Arid3a knockout (KO) mice are embryonic lethal as a result of defects in erythroid lineage differentiation.10 They also have defects in FL HSCs; however, whether AGM HSCs were also impaired was unknown. HSCs do appear in the AGM region of Arid3a KO mice, although with lower frequency than in wild-type (WT) mice. Transplantation using Arid3a KO AGM HSCs shows lower chimerism. Surprisingly, in vivo treatment with IFN-α led to increased levels of Arid3a in adult HSCs,2 whereas pretreatment of Arid3a KO AGM HSCs with IFN-α increased donor chimerism of KO HSCs. These data indicate parallel signaling via IFN-α and Arid3a in AGM HSCs.
Further analysis of Arid3a KO AGM HSCs revealed lower expression of IFNs and IFN receptors and downregulation of IFN target genes, all indicating deficient IFN-α signaling in these cells. The authors then used chromatin-immunoprecipitation sequencing analysis in human hematopoietic cell line K562 and identified overlap between ARID3A and STAT1 binding sites, which implies that ARID3A is a transcriptional co-regulator of STAT1 and that Arid3a and IFN-α–Stat1 act in parallel pathways that converge on Stat1 (see figure).
Together, the data in the article by Kim et al show a role for IFN signaling during hematopoietic development not only at the stage of HSC emergence in the AGM region by IFN-γ and TNF-α, but also during the first maturation steps in AGM HSCs before they migrate to the FL via IFN-α. How the expression of the different IFNs is regulated during normal development and which cells are responsible for the production of IFNs at the different phases of development is likely complex and will require further investigation. In addition, the relationship between Arid3a and IFN signaling is quite complicated and needs to be explored during development as well as in adult HSCs.
Mice lacking the receptors for IFNs or TNF-α are viable, survive to adulthood, and show only limited hematopoietic defects. This indicates that even though these proinflammatory cytokines play an important role in the development of HSCs, reduced signaling does not prevent the development of the hematopoietic system. A current major challenge in this field is to understand the regulation of inflammatory signaling in this new context, from development to adulthood.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal