Abstract
Introduction: Most patients (pts) with diffuse large B cell lymphomas (DLBCL) are cured with first line chemoimmunotherapy including an anthracycline and rituximab. Pts who obtain complete remission (CR) but latter relapse often can be cured with salvage therapy and autologous hematopoietic cell transplantation (auto-HCT). This management paradigm often is applied to pts with primary treatment failure (PTF). However, this group is heterogeneous and detailed data on outcomes in current era is needed to identify the DLBCL pts for whom novel therapeutic strategies should be designed.
Methods: Fifteen US academic medical centers contributed pts to the REgistry of diFfuse large B cell lymphoma with prImary treatmeNt failurE (REFINE) collaboration. REFINE retrospectively captured patient, disease and treatment characteristics and treatment response as assessed by treating physician. Eligible pts were ≥ 18 years diagnosed with DLBCL during 2008-2015, who received upfront cheomoimmunotherapy including anthracycline and CD20-directed antibody and developed one of 3 patterns of PTF: relapse < 6 months following CR (early relapse- ER); only partial remission (PR) or stable disease (SD) with upfront therapy (residual disease-RD); progressive disease (PD) while receiving upfront therapy (primary progression-PP). Pts with HIV infection, primary CNS lymphoma or lymphoma transforming from a more indolent histology were excluded.
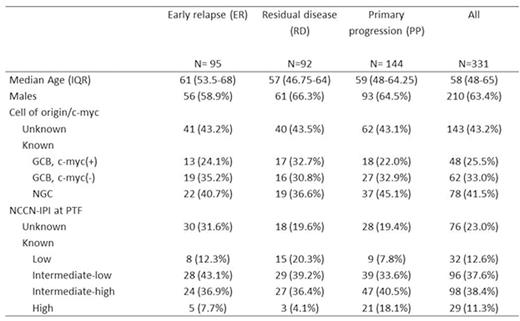
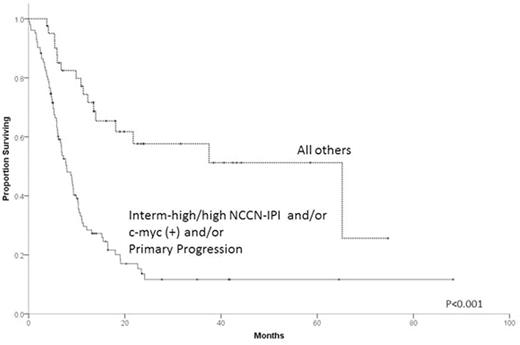
Results: Patient characteristics for the 331 cases are summarized in Table 1. Median follow up of survivors was 18.9 months. R-CHOP was the upfront treatment for 87.6% of pts. Nearly all pts (94.6%) received salvage therapy after PTF and prior to any HCT, with a median of 1 and range 0 to 5 regimens. Response to first salvage regimen was CR in 19.9%, PR in 21.8%, SD in 9.0% and PD in 40.8%. Only 15.1% of pts were enrolled in clinical trials. One hundred and thirty-two pts (39.9%) underwent auto-HCT and 33 (10.0%) allogeneic-HCT (8 after failure of auto-HCT). Two-year overall survival (OS) from time of PTF was 45.5% (95% C.I. 34.5-56.5%) for ER, 30.6% (95% C.I. 20.0-41.2%) for RD and 18.5% (95% C.I. 11.4-25.6%) for PP (P<0.001). Pts with germinal center B cell (GCB), c-myc(+) (by FISH) tumors had 2-year OS of only 11.0% (95% C.I. 0.0-21.6%) vs. 34.9% (95% C.I. 19.4-50.4% ) for GCB , c-myc(-) (P=0.002) vs. 42.3% (95% C.I. 30.9-53.7%) for non-germinal center (NGC) (P=0.01). Two-year OS for NCCN-IPI (assessed at time of PTF) intermediate-high/high risk pts was 10.9% (95% C.I. 4.0-17.8%) vs. 42.3% (95% C.I. 31.3-53.3%) for intermediate-low risk pts and 57.4% (95% C.I. 39.8-75.0%) for low risk pts (P<0.001). Multivariable analysis indicated c-myc(+) and NCCN-IPI at time of PTF as being independent predictors of OS. For 144 pts with complete information on all 3 factors, 2-year OS was 13.6% (95% C.I. 5.8-21.4%) for pts with PP disease, NCCN-IPI intermediate-high/high or c-myc(+), hereafter considered ultra-high-risk (UHR) features vs. 57.6% (95% C.I 40.6-74.7%) for the pts with no UHR features (P<0.001, Figure).
Conclusions: Pts with DLBCL experiencing PTF have poor prognosis but low clinical trial participation even when treated in academic centers. Pts with PP disease, intermediate-high/high NCCN-IPI at time of PTF or with c-myc(+) tumors constitute a UHR category with dismal outcomes on existing therapies. REFINE provides benchmarking for future experimental agents targeting this population. UHR pts represent an unmet medical need and should receive high priority for development of new drugs and cellular therapies.
Chavez:Janssen: Speakers Bureau. Reddy:INFINITY: Membership on an entity's Board of Directors or advisory committees; celgene: Membership on an entity's Board of Directors or advisory committees; KITE: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees. Karmali:Celgene: Speakers Bureau. Calzada:Seattle Genetics: Research Funding. Barta:Janssen: Honoraria, Speakers Bureau; Celgene, Merck, Seattle Genetics: Research Funding. Lansigan:Spectrum: Consultancy, Research Funding; Pharmacyclics: Consultancy; Celgene: Consultancy; Teva: Research Funding. Flowers:Roche: Consultancy, Research Funding; Acerta: Research Funding; TG Therapeutics: Research Funding; NIH: Research Funding; Pharmacyclics, LLC, an AbbVie Company: Research Funding; Infinity: Research Funding; Gilead: Consultancy, Research Funding; Millenium/Takeda: Research Funding; ECOG: Research Funding; AbbVie: Research Funding; Mayo Clinic: Research Funding; Genentech: Consultancy, Research Funding. Fenske:Seatle Genetics: Honoraria; Millennium/Takeda: Research Funding; Celgene: Honoraria; Pharmacyclics: Honoraria. Cohen:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millennium/Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hamadani:Takeda: Research Funding. Costa:Sanofi: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal