Abstract
Background:
EBV+ DLBCL not otherwise specified (EBV+ DLBCL NOS) is defined by the 2016 WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues as EBV+ DLBCL in patients without a history of known immunosuppression. Among Asian patients, adverse outcomes have been associated with EBV+ vs EBV- DLBCL, and circulating EBV has been found in the majority of patients. Outcome studies of unselected North American patients are few and non-confirmatory. No formal assessment of immunosuppression status has been incorporated into these analyses, leading to the variable inclusion of patients with a history of immunocompromised states. We sought to determine the prevalence, presenting clinical and pathology characteristics, and outcomes of DLBCL by EBV and immunosuppression status in an unselected prospective cohort study of North American patients.
Methods:
From 2002-2012, we enrolled consecutive, newly diagnosed patients with lymphoma who were ≥ 18 years and HIV negative into the Molecular Epidemiology Resource (MER). Clinical, laboratory, and treatment data were abstracted from medical records. All DLBCL patients with available tissue microarrays (TMAs), excluding primary mediastinal DLBCL and post-transplant lymphoproliferative disorders, were included. Medical records were reviewed using a standard protocol to classify patients as immunocompromised based on a documented history of primary immunodeficiency or iatrogenic immunosuppression. DLBCL cell of origin was classified as germinal center (GCB) vs non-GCB using the Hans algorithm. CD30 was determined using immunohistochemistry; positivity cutoff was 20% of neoplastic cells. EBV testing was performed by in-situ hybridization for EBV encoded small RNAs (EBERs), and scored by an expert hematopathologist. Event free survival (EFS) was defined as time from diagnosis until recurrence, retreatment, or death due to any cause. Associations with EFS and Overall Survival (OS) were obtained using the methods of Kaplan-Meier (KM). Hazard Ratios (HRs) and 95% confidence intervals (CIs) were estimated from Cox regression models.
Results:
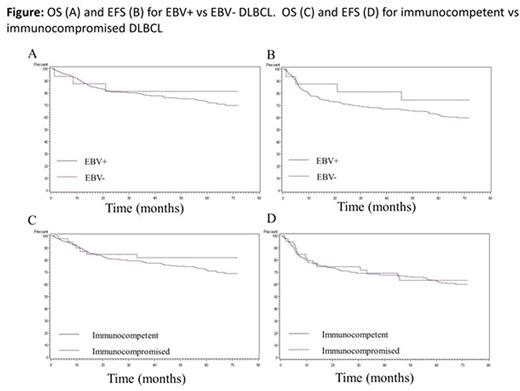
1,100 patients with DLBCL were enrolled into the MER; 362 had TMAs available for this analysis. Median age was 63 years (range 20-89) and 59% were male. 89.5% of patients were treated with immunochemotherapy. 16 cases (4.4%) were EBV+, and 39 cases (10.8%) were considered immunocompromised. EBV positivity was more common in immunocompromised vs immunocompetent patients, with a trend towards significance (10.3% vs 3.7%; P = 0.08). The non-GCB subtype was more frequent in EBV+ vs EBV- DLBCL (62.5% vs 34.1%; P < 0.01), but similar between immunocompromised vs immunocompetent patients (48.7% vs 33.7%; P = 0.10). CD30 positivity was also more frequent in EBV+ vs. EBV- DLBCL (25.0% vs 8.1%; P < 0.01), but similar between immunocompromised vs immunocompetent patients (12.8% vs 8.4%, respectively; P = 0.45). With a median follow-up of 59 months (range 0.7-155.2) there were 151 events and 119 deaths. KM curves by EBV and immunosuppression status are shown in the figure. There was no association of EBV positivity with EFS (HR = 0.76; 95% CI 0.34-1.72) or OS (HR = 0.99; 95% CI 0.43-2.25). A history of immunosuppression was not associated with EFS (HR = 1.17; 95% CI 0.72-1.89) or OS (HR = 1.02; 95% CI 0.58-1.78). In the subset of immunocompetent patients, there was no association of EBV positivity with EFS (HR = 0.54; 95% CI 0.17-1.70) or OS (HR = 0.71; 95% CI 0.23-2.25). Similar results were obtained after adjustment for the International Prognostic Index. Circulating viral DNA was detected in 2/5 EBV+ and 4/49 EBV- DLBCL patients.
Conclusions:
The prevalence of EBV+ DLBCL NOS is <5% among patients in a large North American cohort, similar to other western populations. There was no evidence of an association of EBV or immunosuppression status with EFS or OS, with low power for detecting associations with EBV. Circulating viral DNA was infrequent among either EBV+ or EBV- DLBCL patients. While immunosuppression has been previously shown to be a risk factor for DLBCL, it was not found to be associated with EFS or OS. Overall, these results suggest divergent outcomes among EBV+ DLBCL patients between western and non-western populations, underscoring differences in host factors or circulating viral strains.
Ansell:BMS, Seattle Genetics, Merck, Celldex and Affimed: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal