Abstract
INTRODUCTION:
During the past decades, the outcome of Mantle Cell Lymphoma (MCL) treatment has improved substantially in younger patients. In a recent update of the Nordic MCL2 trial we show very long response durations after a median follow-up of 11.4 years, but we also observe a continuous pattern of relapses even after 10 years of remission.(Eskelund et al, 2016) The course of this disease remains very heterogeneous, and with the current prognostic indexes we are unable to identify patients who might be cured by the current standard-of-care and others who would possibly benefit from alternative frontline approaches. Recently, next-generation sequencing (NGS) studies have explored the mutational landscape of MCL, however, in inhomogeneous and diversely treated cohorts or with short follow-up. Still, TP53 and NOTCH1/2 mutations have been shown to be prognostic markers. In our current study we examine the prognostic impact of aberrations in the most frequently mutated genes in MCL in a homogenously and optimally treated patient cohort, with a long-term follow-up.
MATERIAL AND METHODS:
Freshly frozen DNA from diagnostic bone marrow samples from patients included in two prospective Nordic trials, MCL2 and MCL3 were analysed. In both trials patients received intensified first line induction therapy with alternating courses of R-CHOP and R-HD-Cytarabine and consolidation with high-dose therapy and ASCT. All patients signed an informed consent.(Geisler et al, 2008; Kolstad et al, 2014). NGS was performed using the Ion Torrent Technology. A targeted panel of 8 genes frequently mutated in MCL was constructed on the basis of previous NGS studies.(Bea et al, 2013; Zhang et al, 2014) The panel included all coding regions of the following genes: ATM, CCND1, TP53, KMT2D, NOTCH1, NOTCH2, WHSC1 and BIRC3. Cut-off for calling a mutation was set to a variant allele frequency >3%. Mean depth was >1500X in all patients.
RESULTS:
So far, we have mutational data from 72 patients. Patients were previously untreated and <66 years (median 58, range 37-65). Fifty-three percent were either MIPI intermediate or high risk. Eighty-five percent of patients had bone marrow involvement at diagnosis. After a median follow-up of 11.6 years, median overall (OS) and progression-free survival (PFS) of all 72 patients were 12.4 and 9.8 years, respectively.
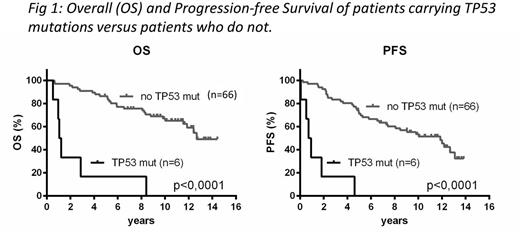
Of the 72 patients, mutations were detected in 33 cases. Twenty-one patients carried 1 mutation and 12 patients had >1 mutation (2-4). Mutations were distributed as follows: ATM 15 (21%), KMT2D 11 (15%), WHSC1 7 (10%), TP53 6 (8%), CCND1 5 (7%), NOTCH2 4 (6%), NOTCH1 3 (4%), BIRC3 2 (3%). In univariate analyses, mutations in TP53 were highly predictive of an inferior outcome (median OS and PFS were 14 and 10 months, respectively; p<0.0001 for both outcomes) (Fig). Likewise, patients with either NOTCH1 or NOTCH2 mutations displayed worse OS (median OS 8.1 years, p=0.023), and there was a trend towards inferior PFS (median PFS 4.5years, p=0.087). No other mutations predicted better or worse outcomes, nor did carrying any one or >1 mutations. In multivariate analyses, the only prognostic significant mutations were TP53 (p<0.0001, HR=15.9) and NOTCH1 (p=0.012, HR=5.3).
TP53 mutations were associated with increased Ki67 expression, median 30% (range 20-80%), higher risk MIPI/MIPI-B/MIPI-C, and 4 out of 6 patients had blastoid morphology at diagnosis. All 6 TP53 mutations were found in the DNA-binding domain, and 5 were missense mutations while the last was a frameshift mutation. Patients with missense mutations all died within 3 years (range 6-34 months) due to relapsing or progressive disease, while PFS and OS of the latter patient was 4.6 years and 8.4 years, respectively.
We found no predictors for late relapses.
CONCLUSION:
Here we evaluate the prognostic impact of mutations in eight genes that are commonly involved in MCL in a cohort of 72 younger patients who received intensified induction therapy and ASCT in the Nordic MCL2 trial. In line with previous reports, we demonstrate a negative prognostic impact of TP53 and NOTCH1/2 mutations; however, in multivariate analyses only TP53 and NOTCH1 mutations held significance. Missense mutations in the DNA binding domain of TP53 seem to predict an exceedingly poor short term outcome. At the annual meeting, we will present mutational analyses and long term follow up of >150 patients from the combined MCL2 and MCL3 cohort.
Jerkeman:Amgen: Research Funding; Gilead: Research Funding; Janssen: Research Funding; Celgene: Research Funding; Mundipharma: Research Funding. Geisler:Roche: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal