Abstract
Background: Gemcitabine is a broadly prescribed chemotherapy, the use of which can be limited by renal adverse events, including hypertension, proteinuria, oedema, acute renal failure and thrombotic microangiopathy (TMA). As opposed to thrombotic thrombocytopenic purpura, gemcitabine-induced TMA generally responds poorly to therapeutic plasma exchange and prognosis is dismal. The usual severe renal involvement and the normal activity of the von Willebrand factor-cleaving protease ADAMTS13 relate gemcitabine-induced TMA to atypical haemolytic syndrome, in which complement blockage is remarkably efficient. In this regard, this study evaluated the efficacy of eculizumab, a monoclonal antibody targeting the terminal complement pathway, in patients with gemcitabine-induced TMA.
Methods: We conducted an observational, retrospective, multicentric study including all patients with gemcitabine-induced TMA treated by eculizumab in 4 French centres, between 2011 and 2014. Patients with a TMA considered to be directly attributed to an uncontrolled cancer were excluded.
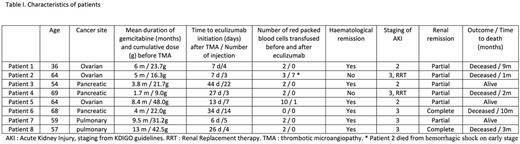
Results: 8 patients with a gemcitabine-induced TMA treated by eculizumab were included (6 women, 2 men). Gemcitabine was prescribed for pancreatic (n=3, 37.5%), ovarian (n=3, 37.5%) and pulmonary (n=2, 25%) cancer. TMA occurred after a median of 5.5 months (range 1.7-13) and a median cumulative dose of 22.8g (range 9.0-48.0). The main characteristics were microangiopathic hemolytic anemia (100%), thrombocytopenia (87.5%), normal ADAMTS13 activity (100%), acute renal failure (100%, including 62% stage 3 acute kidney injury (AKI) and 25% renal replacement therapy), hypertension (75%) and diffuse oedema (62.5%). Eculizumab was started after a median of 19.5 days (range 6-44) following TMA diagnosis. A median of 4.5 injections of eculizumab was performed (range 3-22). Complete haematological remission was achieved in 6 patients (75%) and blood transfusion significantly decreased after only one injection of eculizumab (median of 2 packed red blood cells (range 0-10) before treatment vs 0 (range 0-1) after one injection, p=.015). Two patients recovered completely renal function (25%), and 4 achieved a partial remission (50%), with a median estimated glomerular filtration rate (GFR) improvement of 15 ml/min/1.73m2 (range 7-16). Five patients (62.5%) died during follow-up, from a septic and hemorrhagic shock on early stage (1 case), and from cancer evolution after a median of 6 months (range 2-13) following eculizumab initiation (4 cases).
Conclusion: These encouraging results suggest that eculizumab is efficient on hemolysis and reduces transfusion requirement in gemcitabine-induced TMA. Moreover, eculizumab may improve renal function. Prospective trials are now required to further investigate this issue.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal