Abstract
Introduction: There remains no clear standard first-line therapy for MCL. VcR-CVAD is a novel, intermediate-intensity chemoimmunotherapy regimen which incorporates bortezomib into modified hyper-CVAD chemotherapy. We hypothesized that the addition of bortezomib would improve the complete response (CR) rate, and maintenance rituximab (MR) would improve the remission duration. The results of this study were previously reported (Chang JE, et al. Br J Haematol 2011), with an observed overall response rate (ORR) of 90% (CR/unconfirmed CR in 77%), and 3-year progression-free survival (PFS) and overall survival (OS) of 63% and 86%, respectively. Long-term follow-up (LTFU) is reported from this multicenter trial.
Methods: The study enrolled patients ≥18 years of age with histologically confirmed MCL. Patients were previously untreated, with the exception of 1 cycle of CHOP/CHOP-like chemotherapy. Patients received VcR-CVAD induction chemotherapy every 21 days for 6 cycles: rituximab (R) 375 mg/m2 intravenously (IV) on day 1; bortezomib/Velcade® (Vc) 1.3 mg/m2 IV, days 1 & 4; cyclophosphamide 300 mg/m2 IV every 12 hours, days 1-3 (total of 6 doses); doxorubicin 50 mg/m2 IV continuous infusion days 1-2 (total dose over 48 hours equal to 50 mg/m2); vincristine 1 mg IV day 3; and dexamethasone 40 mg orally days 1-4. Patients received G-CSF support beginning day 5-6 of each induction cycle, and all appropriate supportive care measures were permitted throughout treatment including tumor lysis prophylaxis, transfusion support and antibiotics. Patients achieving at least a partial response to induction therapy received R consolidation (R 375 mg/m2 IV X 4 weekly doses) and MR (R 375 mg/m2 IV every 12 weeks for a total of 5 years; total of 20 doses). Restaging CT scans were performed after cycles 2, 4, and 6 of induction, 12 weeks after consolidation, every 6 months during maintenance, and yearly during LTFU. The primary endpoint was ORR and CR to induction chemotherapy; secondary endpoints were PFS and OS.
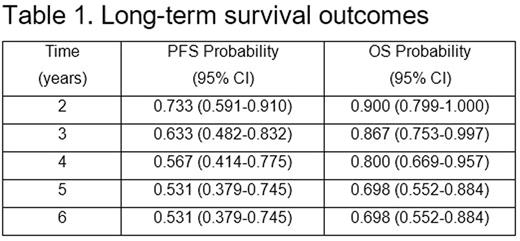
Results: Thirty patients were enrolled from 7/2005-5/2008. Median age was 61 years (range 48-74), 80% male, all patients had advanced stage disease, and 60% had MIPI score of medium- or high-risk disease. Six patients had blastic morphology. Long-term results are reported after a median follow-up of 7.8 years in surviving patients. Twenty patients are alive, and 15 (50%) are alive in ongoing remission (Figure 1). Estimates of 6-year PFS and OS are 53.1% and 69.8%, respectively (Table 1). The observed PFS and OS differences between patients <age 60 and those ≥age 60 were not statistically significant. The observed PFS and OS differences by MIPI score were not statistically significant, although there was a trend towards worse PFS and OS for high-risk MIPI patients. Five patients have died from confirmed progression of MCL. Two deaths occurred from complications post-allogeneic transplant, and 3 deaths occurred from unrelated causes with MCL in remission. No MCL relapses have been observed beyond 5 years. No late toxicities from VcR-CVAD or from MR have emerged during the LTFU.
Conclusions: VcR-CVAD is a moderate intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of MCL. LTFU of patients receiving VcR-CVAD induction followed by 5 years of MR demonstrates high rates of durable remission that are comparable with more intensive chemotherapy and consolidative autologous stem cell transplant (ASCT). The highly promising activity of the VcR-CVAD regimen was recapitulated in ECOG-ACRIN protocol E1405 (Chang et al, Blood 2014). A randomized phase 3 trial has recently confirmed the beneficial effects of bortezomib incorporation into standard immunochemotherapy (Robak et al, NEJM 2015). VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations.
Kahl:Celgene: Consultancy; Seattle Genetics: Consultancy; Infinity: Consultancy; Gilead: Consultancy; Juno: Consultancy; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal