Abstract
Introduction: Acute myeloid leukemia (AML) is a heterogeneous clonal disorder of hematopoietic progenitor cells characterized by excessive proliferation of early progenitor cells in the bone marrow (BM). The BM niche is a specialized type of microenvironment (ME) that actively supports growth, maturation and maintenance of hematopoietic stem and progenitor cells. They include a complex network of extracellular matrix proteins, soluble growth factors, and cytokines. The information regarding the clinical significance of leukemic microenvironment for AML initiation and propagation is scarce. The aim of the current study was to characterize the AML BM ME and its crosstalk with leukemic progenitor cells and define the role of BM stromal cells (SCs) in supporting leukemic cell growth in active disease as compared to the complete remission (CR) state.
Methods: We developed a unique model of patient own stroma (POS) by growing SCs obtained at diagnosis, CR and relapse in a specific medium, including cytokines and proteins derived from human plasma solution. The growth rate and morphological changes of AML SCs were assessed and their ability to support AML cell expansion was studied by monitoring immunophenotyping alterations of CD34 and CD45 expression using FACS analysis. Levels of secreted MCP-1, IL6, GM-CSF, G-CSF and TNF in the co-culture medium were measured using the bead-based immunoassay CBA (BD Biosciences). In addition, real-time PCR (RT-PCR) amplification was performed in the co-culture to quantify changes aiming to assess the expression of specific genes associated with the maintenance of hematopoietic cells, self-renewal, quiescence regulation and mobilization. The following genes were amplified: CXCL12 (SDF1), transforming growth factor beta 1 (TGFβ), secreted phosphoprotein 1 (SPP1) and angiopoietin 1 (ANGP1).
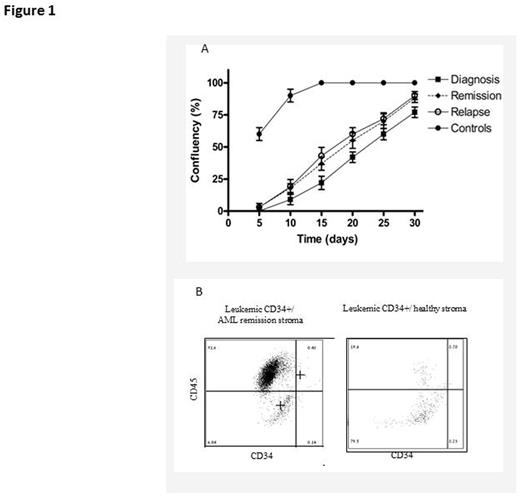
Results: The developed POS model allowed revealing high diversity in the morphological appearance of leukemic SCs obtained from different patients and at various disease stages. Patient SCs were found to grow slower than those of healthy volunteers (fig. 1A). Moreover, the growth rate differed between the patients and disease stages, being highest at relapse and lowest at diagnosis (fig. 1A). Levels of SDF-1, TGF-1 and SPP1 were elevated (X50, X50 and X 300 fold, respectively) in CR stroma compared to that of healthy volunteers. Of note, the level of MCP-1 was increased (2200 pg/ml) in the stroma derived at active disease stages (diagnosis and relapse) compared to CR or normal SCs (1050 pg/ml). Additionally, the expansion of CD34+ leukemic progenitor cells was higher in the presence of CR-derived stroma compared to healthy SCs (fig. 1B). In the co-culture, we observed selective ability of POS to support CD34+ leukemic cell expansion, as detected by flow cytometry (1.5 million cells more in POS than in healthy stroma), as well as differential expression of MCP-1 (x3000 pg/ml) and IL-6 (x9000 pg/ml) cytokines in co-cultures of AML cells and POS. These cytokines are known to be involved in the regulation of cell migration and inflammation.
Conclusions: AML BM SCs are heterogeneous and differ from healthy BM SCs in their growth rate, morphology, immunophenotype features and the ability to support expansion of early progenitor CD34+ cells. Moreover, even an AML CR-derived stroma, which is considered "healthy", can provide an advantageous ME to leukemogenesis, as evident by increased proliferation of CD34+ leukemic cells in a co-culture with CR SCs compared to healthy SCs. Additionally, POS was found to selectively support the expansion of AML cells of the same patient. These findings, suggesting a cross-talk between leukemic progenitor cells and their specific POS, may lead to better understanding of the mechanisms underlying AML initiation and relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal