Abstract
Background. Despite progress in the molecular and genetic classification of pediatric acute myeloid leukemia (AML), the prognosis remains heterogeneous. The ATP-binding cassette transporter A3 (ABCA3) seems specifically involved in the resistance of pediatric AML to intensive chemotherapy. However, studies having investigated the prognostic impact of ABCA3 expression have yielded conflicting results with respect to patient outcomes while the small sample size of these studies precluded the use of multivariate analysis. Here we investigated the prognostic impact of ABCA3 expression in a representative series of homogeneously treated pediatric AML.
Methods. Samples derived from 233 patients with available high-quality RNA and enrolled in the ELAM2 protocol (NCT00149162). qRTPCR amplification of 2 conserved ABCA3 mRNA sequences was performed with GUS and ABL as reference genes. Primer sets were complementary to exons 6-7 and exons 19-20 junctions. Patients were classified according to their standardized cytogenetic and molecular (NPM1 mutations, FLT3-ITD, CEBPA double mutations) risk subgroups (Rubnitz JE, Blood 2012;119:5980-5988, Creutzig U, Blood 2012;120:3187-3205). Treatment consisted of 1 induction course (AraC and mitoxantrone) and 3 consolidation courses (course 1 and 3 with high dose AraC); all children with either intermediate or high-risk disease were candidates for hematopoietic stem cell transplant (HSCT) in complete remission (CR) after 1 to 2 consolidation courses.
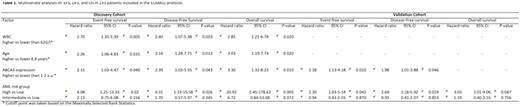
Results. The discovery cohort included 120 patients. Median age, median WBC, CR rate, relapse rate, median follow-up, 5-years EFS, DFS, and OS were 9.4 years, 19.3 G/L, 95%, 29%, 60 months, 58±6%, 61±6%, and 71±5 months, respectively. The two primer sets yielded consistent results (R=0.9, p<10-4, Spearman Rank Correlation). Lower ABCA3 expression was positively associated with CBFB-MYH11 AML (p=0.002) and thereby with favorable cytogenetics (p=0.036) and low-risk AML (p=0.027). Higher ABCA3 expression was associated with higher relapse rate (p=0.006), shorter EFS (5-years, 34±9 vs 61±6 % p=0.0005), DFS (36±9 vs 62±6% p=0.0028), and OS (49±12 vs 79.5±5% p=0.0007). Multivariate analyses identified age, WBC, risk group, and ABCA3 expression as independent prognostic factors for EFS, DFS, and OS (Table 1). The validation cohort included 113 patients in whom the proportions of AML1-ETO- and MLL-positive AML were significantly higher than in the discovery cohort: 26,5% vs 6,7% (p<10-4) and 24.8 vs 14.2% (p=0.03). There was no significant difference in patients' outcome between the 2 cohorts. Using the same cutoff value in the validation cohort, higher ABCA3 expression remained significantly associated with shorter 5-years EFS: 63±7% vs 43±9% (p=0.025) with a trend for shorter DFS: 45±9 vs 53±11% (p=0.065). Multivariate analyses identified ABCA3 expression as an independent negative prognostic factor for EFS and DFS (Table 1). In the entire patients population, ABCA3 expression independently predicted EFS, DFS, and OS (not shown). In the low- (n=74) and adverse-risk (n=59) groups, higher ABCA3 expression remained associated with shorter 5-years EFS (low: 46±12 vs 75±7%, p=0.006; adverse: 12±10 vs 44±16%, p=0.018), DFS (low: 49±13 vs 75±7%; high: 12±11 vs 45±16%, p=0.016), and OS (low: 76±10 vs 94±4%; adverse: 32±14 vs 57±18%, p=0.046).
Conclusion. ABCA3 expression represents an independent prognostic factor in pediatric AML. As they indicate that the level of ABCA3 expression is significantly associated with survival for currently accepted cytogenetic and molecular prognostic categories, our findings suggest that assessing ABCA3 expression will permit a better assessment of disease risk. Finally our results suggest that inhibiting ABCA3 expression, such as with indomethacin, could be beneficial in order to overcome drug resistance in pediatric AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal