Abstract
Introduction: Leukemic sub-clones which survive chemotherapy during induction period may remain dormant and subsequently lead to relapse in patients with acute myeloid leukemia (AML). No mechanism linking clonal selection to a specific leukemia-associated genetic aberration is known. Clonal selection is a common phenomenon; therefore, any suggested mechanism should be applicable to all kinds of leukemia-specific genetic aberrations. We hypothesized that clonal selection might result from differences in differentiation capacity among sub-clones and that chemo-resistance is not necessarily directly affected by specific mutations.
Patients and Methods: We tested this hypothesis in vitro using the kasumi-1 leukemia cell line, which undergoes limited differentiation while growing and is composed of both CD34+ and CD34- sub-populations. Kasumi-1 cells were sorted by fluorescence-activated cell sorting (FACS-Aria IIIu, BD Biosciences) to CD34+CD117+ and CD34-CD117+ sub-populations. Each sub-population was separately exposed to escalating doses of daunorubicin (DNR) and cytarabine (ARA-C) for 66 hours. Cell viability was determined by alamarBlue assay (Bio-Rad, USA); apoptosis was assessed by Annexin-V and propidium iodide (PI) staining. In vivo experiments included BM samples derived from three AML patients, with both NPM1 and FLT3 gene mutations, who received intensive induction and subsequently relapsed. Blast cells were sorted to CD34+CD117+ and CD34-CD117+ sub-populations. Targeted gene sequencing was conducted using Ion Torrent™ Personal Genome Machine® (PGM) System (Life Technologies, USA). Allelic frequency of NPM1 and DNMT3A mutations in CD34+ and CD34-sub-populations was measured. FLT3-ITD was sequenced using theGeneScan method and theallelic ratio (AR) was calculated.
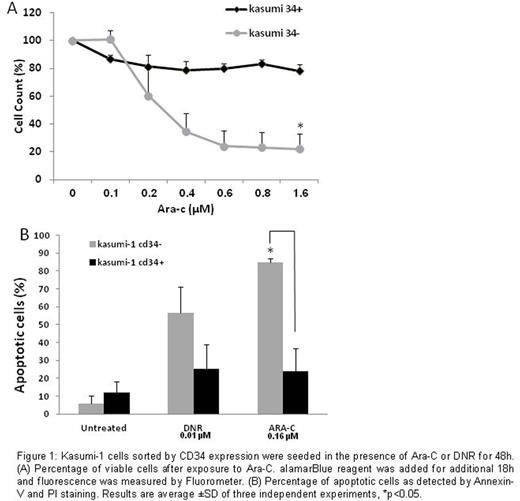
Results: Following sorting,cultured kasumi-1 CD34+ cells partly differentiated into CD34- cells and several weeks later, the phenotype became similar to the original kasumi-1 phenotype (a mixture of 34+/- cells). In contrast, the CD34- sub-population maintained its phenotype. Immediately after sorting by CD34 expression, both sub-populations were exposed to increasing doses of DNR or ARA-C. Only the CD34+ sub-population was found to be resistant to ARA-C (at all tested concentrations), as determined by the cell viability assay (fig 1a). The CD34- sub-population exhibited a 4-fold higher apoptosis rate than CD34+ cells after exposure to 0.16 µM ARA-C. DNR (0.01 µM) resulted in 2.5-fold higher apoptosis in the CD34- compared to CD34+ sub-population (fig 1b).
The sub-clonal composition of BM blasts obtained from three AML patients at diagnosis and relapse was compared. NPM1 mutation was identified in all patients at diagnosis. FLT3-ITD mutation was identified in one patient at diagnosis and in the other two at relapse. To explore the differentiation capacity of each sub-clone, blasts were sorted to well-defined CD34+ and CD34- subgroups. At diagnosis, most of the leukemic blasts presented with the CD34- phenotype, while at relapse, the CD34+ population grew in all patients. The FLT3-ITD allelic ratio was higher in CD34+ cells. In the first patient, FLT3-ITD was not detected at diagnosis, while at relapse, the wild-type allele of FLT3 was lost and FLT3-ITD was solely present in the CD34+ sub-population. In CD34- cells, the AR was low (0.55) at relapse. In the second patient, the dominant clone at diagnosis was NPM1mutFLT3wtDNMT3Awt and >95% cells were CD34-. At relapse, the FLT3-ITD DNMT3Awt clone became dominant and was mostly located within the rising CD34+ population. Another sub-clone, exhibiting both FLT3-ITD and DNMT3A R882C mutation, was identified and was found to reside only within the CD34+ population. Similar findings were also observed in the third patient. We screened additional eight patients presenting with FLT3-ITD and in 4 of them, while both CD34+/- blasts were observed, the FLT3-ITD carrying clones resided only in one sub-population.
Conclusions: Chemo-resistance and survival of a specific AML sub-clone correlate with its phenotype and differentiation level. Even genetic aberrations which have no direct interaction with chemotherapy biological effect, may give rise to clonal selection. Sub-clonal differentiation capacity may affect its sensitivity to chemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal