Abstract
BACKGROUND:
Lymphodepletion chemotherapy with infusion of CD19-specific chimeric antigen receptor (CAR)-modified T cells has produced impressive antitumor responses in CD19+ malignancies in phase 1 clinical trials, but can be associated with cytokine release syndrome (CRS) and neurotoxicity (NT). Our understanding of CRS and NT continues to evolve, and identification of predictive biomarkers and strategies to mitigate these toxicities will facilitate management of patients (pts) undergoing CD19 CAR-T cell therapy in multicenter phase 2 trials.
METHODS:
We treated 127 adults with ALL, NHL or CLL with anti-CD19 CAR-T cells manufactured from defined CD4+ and CD8+ T cell subsets, formulated in a 1:1 ratio of CD8+:CD4+ CAR+ T cells, and infused at 1 of 3 dose levels (2x105, 2x106 or 2x107 CAR-T cells/kg) following lymphodepletion chemotherapy. The incidence and grade (gr) of CRS and NT, and correlative biomarkers were analyzed through 28 days after infusion.
RESULTS:
One hundred and nine pts (45 ALL, 47 NHL, 17 CLL) have completed toxicity and response assessment. CRS was graded according to Lee et al (Blood, 2014) and developed in 71% of pts (44% gr 1-2, 22% gr 3-4, 5% gr 5). Pressors were only required in 21% of the pts who developed gr 3-4 CRS. Gr ³3 NT (CTCAE v 4.03) developed in 25% of pts, all of whom developed fever before NT. The median duration of all-cause hospitalization from the start of lymphodepletion was 7, 6, and 10 days for ALL, NHL and CLL pts, respectively.
Because we observed short CAR-T cell persistence and an anti-CAR transgene immune response in an initial cohort of pts who received cyclophosphamide (Cy) lymphodepletion, fludarabine (Flu) was added to Cy. This regimen abrogated immune CAR-T cell rejection and dramatically increased early CAR-T cell expansion. We found that infusion of 2x107 CAR-T cells/kg after Cy/Flu was excessively toxic (5/9 developed gr 4-5 CRS), and identified 2x106 CAR-T cells/kg as the maximum tolerated dose in NHL and CLL. Of 26 NHL pts (22 with aggressive histology) treated with Cy/Flu and 2x106 CAR-T cells/kg, the ORR was 73% and the CR rate 46%. No pts had gr 5 CRS or required pressors, and only 12% of pts experienced either gr 3-4 CRS and/or gr ³3 NT. Of 13 CLL pts treated with Cy/Flu and ²2x106 CAR-T cells/kg, the ORR (CT+/-PET) was 85%. In 85%, of pts no marrow disease was detected by flow cytometry. Overall, 38% of pts achieved CR, including 1 pt with residual CLL after the first infusion who achieved CR after a second CAR-T cell infusion. No pts had gr 4-5 CRS or required pressors, and 23% had either gr 3 CRS and/or gr 3 NT.
In ALL pts, the incidence of CRS and NT correlated with the percentage of marrow blasts and CAR-T cell dose, and further dose modification was required for pts with ³ 5% marrow blasts, in whom 2x106 CAR-T cells/kg was excessively toxic (56% gr 4-5 CRS; 56% gr 4-5 NT; n=9). CRS and NT was mitigated in pts with ³ 5% blasts by administering 2x105 CAR-T cells/kg resulting in 6% gr 4 CRS, 17% gr 3; and 17% gr 3-4 NT; n=18). Efficacy was not compromised with the T cell dose reduction for the high tumor burden cohort with 89% of pts achieving a bone marrow CR by high-resolution flow cytometry.
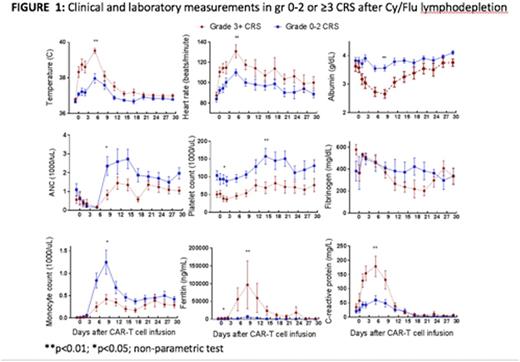
Detailed characterization of early biomarkers of CRS and NT may provide an opportunity to develop data-driven toxicity grading to facilitate mitigation strategies that could be applied across different centers. Compared to pts with gr 0-2 CRS, those with gr 3-5 CRS had significantly higher peak levels of IL-15, IL-6, IL-2, IFN-g, C-reactive protein, and ferritin in both ALL and NHL cohorts. Importantly, in univariate analysis, the levels of IL-15, IL-6, IL-8, IL-10, soluble TNF receptor type 1, and IFN-g were significantly higher on day 1 after CAR-T cells in pts that developed gr 3-5 CRS, and might be used to identify pts to test early intervention strategies to reduce later severe toxicity. We made similar observations in pts with and without NT. Multivariate analysis of biomarkers including clinical and laboratory parameters (Figure 1) is ongoing.
CONCLUSION:
CD19 CAR-T cell immunotherapy can be associated with severe CRS and NT. The use of CAR-T cell products with a prescribed 1:1 CD4/CD8 composition identified CAR-T cell doses associated with a reduced incidence and severity of these complications without impairing efficacy. Additional study of correlative biomarkers to inform rational strategies for early intervention will facilitate the safe and effective clinical application of CAR-T cell therapy.
Turtle:Seattle Genetics: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Research Funding. Li:Juno Therapeutics: Employment, Equity Ownership. Riddell:Juno Therapeutics: Equity Ownership, Patents & Royalties, Research Funding; Cell Medica: Consultancy, Honoraria; Adaptive Biotechnologies: Consultancy, Honoraria. Maloney:Juno Therapeutics: Research Funding; Genentech/Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal